Research
Highlights...
|
|
Fermilab's
Elizabeth Freeland
|
|
 |
Number 194 |
October 10, 2005 |
|
Ames chemists resolve century-old controversy
DOE Ames Laboratory senior chemist Andreja Bakac and assistant scientist Oleg Pestovsky have resolved the 100-year-old debate over the mechanism that triggers one of the most powerful oxidizing reactions available for breaking apart organic molecules. They have generated, characterized and ruled out iron (IV) as the crucial intermediate in the Fenton reaction, a complex and pervasive reaction in matters associated with biological systems, environmental and atmospheric processes, and catalytic chemistry. Their indisputable research results establish hydroxyl radicals (OH radicals) as the crucial Fenton intermediates. The illuminating work was done in collaboration with researchers at Carnegie Mellon University in Pittsburgh and the University of Minnesota in Minneapolis.
[Saren Johnston, 515/294-3474,
sarenj@ameslab.gov]
|
Spinning quarks
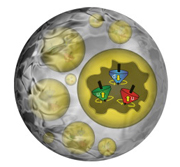 |
| An artist's depiction of quarks as spinning tops inside protons, with the protons embedded in a nucleus.
|
Just as a top spins on a table, the tiny quarks inside protons and neutrons (nucleons) also spin. Now a complex calculation by theoretical nuclear physicists at DOE's Jefferson Lab has revealed that a quark's spin may be altered by the surroundings of the nucleon in which it resides. The surprising result comes from a calculation of the spin-dependent structure functions for nucleons inside the nucleus and for free nucleons - those outside the nucleus. The structure functions provide information on how the quark spins are organized inside the nucleon. The research has been published in Physical Review Letters [Kandice Carter, 757/269-7263,
kcarter@jlab.org]
|
|
Argonne helps 'power' protection for soldiers
Next-generation soldiers will wear vests with a battery to power the many high-tech devices that modern soldiers use in battle. DOE's Argonne National Laboratory is developing the materials and cell chemistry for that battery. Modern military personnel rely on non-rechargeable batteries to power communications, night vision goggles and global-positioning sensors. In an ongoing project for the U.S. Army Communications-Electronic Research & Engineering Center, Argonne is developing battery chemistry for Quallion LLC's battery pack for the Power Vest. DOE's Office of Energy Efficiency and Renewable Energy provided the original funding to Argonne in 1998 to improve safety and calendar life for these energy-storage devices.
[Catherine Foster, 630/252-5580
cfoster@anl.gov]
|
|
Portable nuclear material detection system
Researchers at DOE's Savannah River National Laboratory have developed a lightweight, portable system that can rapidly detect the presence of nuclear materials in sealed containers. Using sensors arrayed linearly and encased in fabric, the RadRope system can be dangled in the small gap between stacked shipping containers on a cargo ship. As the inspector walks along the top containers, a hand-held PDA shows an alarm when any sensor in the array detects radiation levels above background radiation. The system, which can be easily used by one person, detects gamma and neutron radiation. The length of the RadRope system can be easily customized for different uses. It can also be used in a curved or angled line; for example, it can be mounted on a frame through which items of interest are passed.
[Angeline French, 803/725-2854,
Angeline.French@srs.gov]
|
|
|
Freeland overcomes hurdles of physics career breaks
 |
Elizabeth Freeland, mother of two, works with the Lattice QCD group at Fermilab. |
Elizabeth Freeland took a long road to arrive at crunching numbers for Lattice QCD in the Theoretical Physics Department of the Department of Energy's Fermilab. After receiving her doctorate in condensed-matter physics from Johns Hopkins University in 1996, Freeland took a five-year career break for motherhood before turning back to the field. She was met with a series of hurdles built by her absence. “There's this mindset that if you take time to do anything but physics, then you're not serious,” Freeland said.
Research opportunities required a grant. Grants required her to have a full-time affiliation with less than a five-year break after graduate school. As a mother of two and a part-time physics teacher at the School of the Art Institute of Chicago, Freeland had neither. “If I couldn't get a grant, I couldn't have day care, and if I couldn't get day care, I couldn't do the work,” she said.
Freeland eventually earned enough money from teaching to pay for day care while conducting part-time research at Fermilab, which she said has been supportive of her situation. Then she found a grant from the American Association of University Women for which she was eligible. Her application was accepted, and the one-year grant allowed her to start at the lab full-time in July.
Freeland, who explores the issue on her personal website says the traditional method of awarding grants and hiring, as well as cultural attitudes toward career breaks, sets many women up for failure. “You should be able to sit down at a lunch table and say ‘When is a good time to have children or how can I deal with this?'” she said. “You should be able to ask that question to a group of physicists and not have it looked at as a negative.”
Submitted by DOE's
Fermilab
|
|




