| Research
|
Today, the researcher at DOE's Savannah River National Laboratory is a nationally recognized remote thermal IR expert. Washington Savannah River Company, which operates the Savannah River Site and its National Laboratory for DOE, honored him for the way he makes use of those opportunities by presenting him with the Don Orth Award of Merit. The Orth Award, named for an internationally known nuclear chemist who retired from SRS in 1992, is SRS' highest honor in engineering and technical leadership. Dr. Garrett, who earned a Master's degree in Meteorology (MIT) and a PhD in Civil Engineering (University of Texas), has been at SRS since 1979, first joining SRNL as a meteorologist. His experience as manager of the Meteorology Group, combined with his knowledge of fluid dynamics, led to management assignments in the Savannah River Site's reactor safety programs. When the reactors were shut down, he decided to return to technical laboratory work. The increasingly complex world situation led him to work in remote sensing, where he could make the most beneficial use of his technical background in meteorology, numerical modeling, and reactor operations. He developed a thermal analysis code that is recognized by U.S. federal agencies, universities, and commercial power companies for security, research, and operational assessment. He was also the SRNL lead in its function as the ground truth collections lab for DOE's Multispectral Thermal Imaging (MTI) satellite, which was designed and built by Sandia National Laboratories and Los Alamos National Laboratory. Dr. Garrett's technical leadership has contributed to the recognition of SRNL as a leader in remote sensing and as a major contributor to national security. He continues to collaborate extensively with other national laboratories, government agencies, and various universities.Submitted by DOE's Savannah River National Laboratory |
||||||||||||||||||||||||||||
|
Check out the Office of Science's new Website.
|
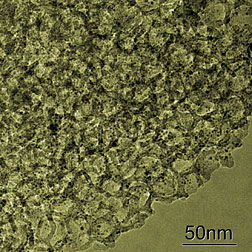
An insulating breakthrough
A new insulating material with the lowest thermal conductivity ever measured for a fully dense solid has been created at the University of Oregon (UO) and tested at DOE's Advanced Photon Source at Argonne National Laboratory. The research was carried out by collaborators from the UO, the University of Illinois at Urbana-Champaign, the Rensselaer Polytechnic Institute, and Argonne. The principles involved, once understood, could lead to improved insulation for a wide variety of uses, the scientists say. "The reason for the extraordinarily low thermal conductivity that we've now achieved is an unusual structure which is crystalline in two directions but has a subtle rotational disorder in the direction of low-heat conduction," David C. Johnson, a professor of chemistry at the University of Oregon and member of the UO Materials Science Institute. The material prepared in Johnson's lab "is the closest thing that anyone has found to making a dense solid into a perfect thermal insulator," said co-author and corresponding investigator David G. Cahill, a professor of materials science and engineering at the University of Illinois at Urbana-Champaign. "This new physical properties displayed by this material might some day point the way toward methods of creating more effective practical insulations." "Thermal conductivity is an important property in both conserving energy and in converting between forms of energy," Johnson said. "Obtaining low thermal conductivity in a thermoelectric material, which converts temperature gradients into electrical energy, increases efficiency." The properties of Johnson's material were measured in Cahill's Illinois laboratory. The structure was analyzed at the APS. Computational simulations and molecular modeling of the layered crystals was carried out by researchers at Rensselaer Polytechnic Institute (RPI) in Troy, N.Y. Submitted by DOE's Argonne National Laboratory |