- Number 311 |
- May 10, 2010
Artificial diamonds may make fuel cells more affordable
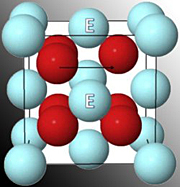
Oxygen (red spheres) migrates
from one vacancy to another
inside the scandia-doped cubic
zirconia. The cations the
oxygen must brush past are
marked by the letter E.
Using specialized cubic zirconia or artificial diamonds, scientists from Nanjing Normal University in China and DOE’s Pacific Northwest National Laboratory created a membrane that could drop the temperature inside solid oxide fuel cells (SOFCs). Lowering the temperature means these cells could be built from less expensive materials. Currently, the temperature inside SOFCs is about 1000 degrees Celsius. With this much heat, the cells must be constructed using very durable, very expensive ceramics. Lower temperatures mean the cells could be built from inexpensive stainless steel. The trick to dropping the temperature, and thus the cost, is the membrane at the heart of the cell. The team’s new scandia-doped cubic zirconia can work at temperatures as low as 650°C. This work was done at DOE’s EMSL, a national scientific user facility.
[Kristin Manke, 509.372.6011,
kristin.manke@pnl.gov]
