- Number 312 |
- May 24, 2010
Detangling algae for future fuels
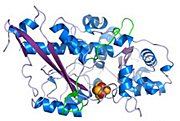
Structure of a unique enzyme
that naturally produces hydrogen
gas.
Environmentalists dream of a world that runs on hydrogen, but cranking out the gas is expensive and often uses precious metals. But now scientists conducting research at DOE's SLAC National Accelerator Laboratory's Stanford Synchrotron Radiation Lightsource have uncovered insights into how a complicated enzyme that naturally produces hydrogen gas is assembled in nature. The finding could be a key step in understanding how microorganisms could cheaply mass-produce hydrogen for energy sources such as fuel cells.
Enzymes, proteins that jump-start chemical reactions, are found in microorganisms all over the planet, turning nitrogen into life-essential amino acids, converting carbon dioxide from gas to solid, and giving off hydrogen. Scientists knew a particular hydrogen-producing enzyme, found in algae, worked by shuffling around iron and sulfur, but didn't know specific about how they are assembled in the enzyme.
"If you want to genetically engineer algae to make more of an enzyme and mass-produce it, you need to know how it is assembled," said John Peters, a chemist at Montana State University who conducted the experiments at SSRL.
Under flashes of SSRL's X-ray light, Peters and his team took microscopic snapshots of the enzyme. By watching X-rays diffract from crystals of the enzyme, the chemists concluded that clusters of iron are assembled step by step to make the active hydrogen producing enzyme.
The insights may help scientists build something that mimics the biological structure and make their own synthetic enzyme, or mass-produce algae with large amounts of the enzyme in giant pools.
Learn more… (http://www-ssrl.slac.stanford.edu/newsletters/headlines/headlines.html#Highlight1)[Kelen Tuttle, 650.926.2585,
kelen.tuttle@slac.stanford.edu]
