- Number 333 |
- March 21, 2011
How metal organic framework sorbents adsorb CO2
How metal organic framework
sorbents adsorb CO2.
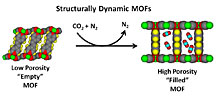
Scientists from DOE’s National Energy Technology Laboratory and the U.S. Department of Commerce’s National Institute of Standards and Technology have been collaborating to determine how structurally dynamic sorbents rearrange their atoms to selectively adsorb CO2 from mixed gas streams containing CH4 and N2.
The NETL-NIST team has used neutron diffraction and neutron vibrational spectroscopy to determine changes that occur in the positions and motions of atoms when CO2 is adsorbed by a structurally dynamic metal organic framework (MOF) sorbent. A third technique, called small angle neutron scattering, was used to study how the sorbent particle’s size, shape, and packing density changed as a result of these atomic-level structural changes.
The data indicates that the atomic sheets in the sorbent apparently exhibit a breathing motion that is initiated specifically by the CO2 molecule in mixed streams containing N2, CH4, and other gases. This atomic level breathing motion causes the macroscopic size of the sorbent particles to increase by up to 50%, illustrating a link between atomic scale phase transitions and more practical engineering considerations such as particle morphology and sorbent bed packing density.
An unusual behavior was also noted when the sorbents released the CO2 involving a complex, multiphase, sorbent structure unique to the desorption process. This observation helps explain a well-known desorption hysteresis noted in the isotherms of these MOFs. Understanding this hysteresis will enable engineers to design improved pressure swing adsorption cycles for these sorbents.
[Linda Morton, 304.285.4543,
Linda.morton@netl.doe.gov]
