- Number 370 |
- August 27, 2012
NETL, partners collaborate in development of ionic liquids for carbon capture
First-of-its-kind membrane screening
system.
The capture and sequestration of carbon dioxide from fossil-based power generation is a vital step in the ongoing efforts to halt the rise of atmospheric CO2 levels. Conventional carbon capture technologies are both inefficient and prohibitively expensive. Improvements in both capture materials and processes will be required if carbon capture and sequestration is ever to become economically feasible. Researchers at DOE’s National Energy Technology Laboratory (NETL) have obtained promising results with a unique class of materials called ionic liquids. In collaboration with its Regional University Alliance (RUA), University of California, Berkeley, and Lawrence Berkeley National Laboratory, NETL is developing these materials as part of a larger effort to develop improved carbon capture technology.
The NETL-RUA has unique capabilities that enable the development of technologies for carbon capture. The alliance is comprised of three ORD research facilities in Albany, OR; Morgantown, WV; and Pittsburgh, PA; partnered with several regional academic institutions (Carnegie Mellon University, University of Pittsburgh, West Virginia University, Penn State University, and Virginia Tech). Through the NETL-RUA, NETL is able to call upon the huge array of facilities and expertise from the regional universities, and the universities gain the benefits of NETL's unique testing facilities and unrivaled expertise in CO2 capture from power systems including pulverized coal combustion, integrated gasification combined cycle (IGCC), and oxy-fuel combustion plants. It is the combination of this impressive array of facilities, expertise, and focus on finding a solution to the CO2 problem that has allowed the NETL-RUA to develop its integrated technology development approach. Integrated technology development brings together expertise in molecular simulation, synthesis, fabrication, characterization, performance testing, and systems design to rapidly develop new carbon capture concepts from ideas into technologies.
Traditionally, three approaches have been utilized to separate CO2 from the gas byproducts in power plants: sorbents, membranes, and solvents. Sorbents (solids) and solvents (liquids) are materials which, when in contact with gas mixtures, selectively adsorb CO2. These materials then release the captured carbon after an alteration in temperature or pressure, allowing the collection of the CO2 and the reuse of the material. A membrane is a barrier that induces separation through selective permeation. Solvents are the best understood of these technologies, and the commercially available products for CO2 capture are solvent based.
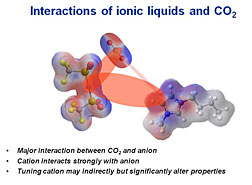
Ionic liquids are a unique class of solvents being studied by the NETL-RUA. An ionic liquid, or IL, is a salt in the liquid state near ambient conditions. Ionic liquids have unique properties that make them desirable for many chemical and commercial applications: low vapor pressure, non-flammability, a wide liquid temperature range, a wide electrochemical window, and high thermal stability. Further, as a result of the nearly infinite variety of available ionic liquid structures, it should be possible to precisely tailor the properties of these materials for CO2 capture.
It is this abundance of potential ionic liquids, an estimated 1018 possible structures, that has led researchers to adapt the integrated technology development approach to allow for an even greater degree of computational screening through the use of chemical informatics. Combining molecular simulation and cheminformatics from the University of California, Berkeley/LBNL with experimental results from the NETL-RUA, researchers are able to search huge numbers of possible materials for a particular set of desirable properties. This approach provides a screening tool that allows researchers to selectively focus their time and resources effectively by indicating the most promising materials.
In addition to utilizing ILs as conventional solvents, it is also possible to convert them into membranes. Currently available supported ionic liquid membranes (SILMS) have demonstrably high CO2 permeability compared to most other types of membranes. However, these membranes have traditionally been flat films with unrealistic mechanical properties, problems which have kept them confined to the laboratory and out of commercial applications. NETL-RUA researchers have been developing these membranes in a hollow fiber configuration which leads to a smaller footprint, allows higher efficiency module design, and lowers fabrication cost. Polymer fabrication experts have already succeeded in creating ionic liquid—containing hollow fibers with permeances near those of commercial membranes. Further developments in module fabrication are expected to move supported liquid membranes toward their first commercial applications.
The rate of advance in the creation of new membrane materials has made it nearly impossible to keep up with performance testing. To address this issue, the NETL-RUA has created a first-of-its-kind membrane screening system capable of measuring the mixed gas performance of 16 membranes simultaneously. In conjunction with the existing facilities and expertise available through the NETL-RUA, the new rapid membrane screening capability is expected to drive further advancements in carbon capture technology.Submitted by DOE’s National Energy Technology Laboratory
