- Number 416 |
- June 23, 2014
Details of calcium 'safety-valve' in cells could lead to new cancer-fighting drugs
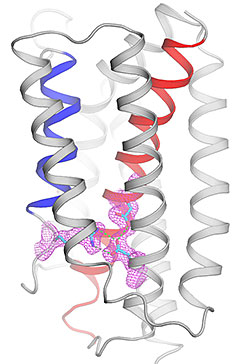
Viewed from within the membrane, the
structure of a calcium-leak channel is shown
as ribbons in a closed-conformation. At
physiological pH, open and closed
conformations exist in equilibrium,
maintaining a steady of state of calcium in
the cell by allowing gradual leakage of
calcium through a transient transmembrane
pore.
Sometimes a cell has to die—when it's done with its job or inflicted with an injury that could otherwise harm an organism. Conversely, cells that refuse to die when expected can lead to cancer. So scientists interested in fighting cancer have been keenly interested in learning the details of "programmed cell death." They want to understand what happens when this process goes awry and identify new targets for anticancer drugs.
The details of one such target have just been identified by a group of scientists from DOE's Brookhaven Lab, Columbia University, New York University, Baylor College of Medicine, Technical University of Munich, and the New York Structural Biology Center. The group, known as the New York Consortium on Membrane Protein Structure (NYCOMPS), used x-rays at Brookhaven Lab's National Synchrotron Light Source (NSLS) to decipher the atomic level structure of a protein that regulates the level of calcium in cells.
"The accumulation of calcium is a key signaling agent that can trigger programmed cell death, or apoptosis," explained Wayne Hendrickson of Columbia and Brookhaven, and the director of NYCOMPS, as well as a senior author on a research article describing the findings in Science June 6, 2014. "Our study reveals how this protein serves as a molecular safety valve for keeping calcium levels steady. Designing drugs that inhibit this protein would promote cell death, which could be a promising strategy for fighting cancers in which such proteins are overexpressed"—including prostate, breast, glioma, uterine, ovarian, and lung cancers.
"Our work, using the prokaryotic version of this protein, has enabled us to construct a three-dimensional model that can be used as a basis for the rational design of possible inhibitor molecules," said Qun Liu, a scientist at NSLS and NYCOMPS and the lead author on the paper.
Next the scientists will study human versions of the proteins to refine the design of possible inhibitor drugs using x-ray beams 10,000 times brighter at Brookhaven’s soon-to-be-complete new light source, NSLS-II.[Karen McNulty Walsh, 631.344.8350,
kmcnulty@bnl.gov]
