- Number 430 |
- January 12, 2015
Electron-rich ions retain charge when softly landed
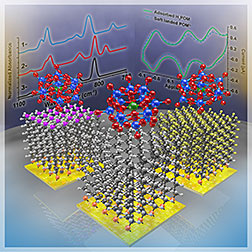
Scientists determined that negatively
charged Keggin ions, shown resting atop
carbon-based surfaces, do not lose their
charge when softly landed.
Creating extended range electric cars and high-capacity flash memory for portable electronics requires scientists to delve into the behavior of anions, negatively charged molecules, that can store extra electrons needed to get the job done. For the first time, scientists at DOE's Pacific Northwest National Laboratory (PNNL) determined how carefully prepared electron-rich anions interact with three well-known carbon-based surfaces. Unlike positively charged ions, anions retain their charge and fail to transfer electrons to the surface. The Keggin anions refuse to release their electrons to the surface because of the substantial force holding the electrons to the molecule.
"In contrast with positive ions, charge retention by negative ions is less surface dependent," said Dr. Julia Laskin, a PNNL Laboratory Fellow who led the research. "In this case, the properties of the ion determine whether it retains its charge on the surface."
This study provides fundamental insights into polyoxometalate anions that may help control their performance in supercapacitors to create improved devices for cars and portable electronics with more charge capacity (and hence more energy). "Research in supercapacitors and molecular electronics will benefit from understanding the charge retention of ions at the nanometer scale," said Dr. Grant Johnson, a physical chemist at PNNL who participated in this study. "In addition, there are potential opportunities for charged species in catalysis and chemical sensors."
Now, Laskin and her colleagues are studying how soft landing of tungstate-based polyoxometalates may improve the capacitance or electrochemical energy storage of electrodes. The tungsten material is structurally more robust than the molybdenum studied here and may provide key insights needed to design high-performance supercapacitors.
This research was funded by the U.S. Department of EnergyOffice of Science's Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division.[Kristin Manke, 509.372.6011,
kristin.manke@pnl.gov]
