 |
| David Geohegan (left) and Alex Puretzky capture digitized snapshots of the vaporized plume of carbon species created during laser ablation of pyrolytic graphite to synthesize new materials. An intensified charge-coupled detector array camera is used in fundamental studies of the target vaporization, vapor transport, and deposition processes that occur during only a few microseconds. The studies are used to optimize vacuum growth of transparent films of amorphous carbon with the properties of diamond, or the growth of clusters in background gases to useful nanoparticles (e.g., buckyballs). Photograph by Tom Cerniglio |
Laser-Deposited Films Improved with Aid of Plume Pictures
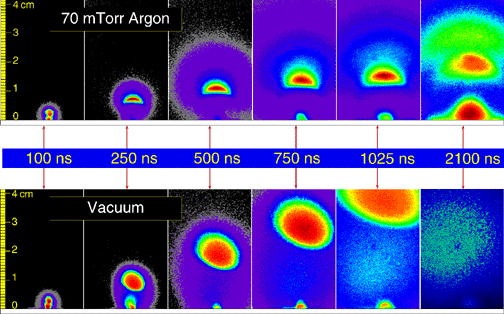 |
| Stop-action photographs reveal how a plasma plume composed of laser-vaporized carbon ions, atoms, and clusters moves away from a graphite surface into vacuum (bottom) and into a low-pressure background gas (70-mTorr argon, top). The digital images of the plasma light are taken at different times after the vaporizing laser pulse and are assigned false colors to reveal different regions of emission for spectroscopic identification. Expanding into vacuum (bottom sequence), the bright ball of fast carbon ions responsible for high-quality amorphous diamond films is the dominant plume component, while slower atoms and clusters are barely visible. However, the top image sequence shows how a low-pressure gas scatters and slows these ions, confining the plume so that carbon-carbon collisions can form clusters, such as buckyballs, near the target surface for fabrication of novel materials in the gas phase. |
 ..... from the 1998 "State of the Laboratory" issue of the ORNL Review
..... from the 1998 "State of the Laboratory" issue of the ORNL Review
...touch for larger version
Just as solar rays turn comet ice into a blazing, cloudlike tail, pulsed beams of ultraviolet laser light can rapidly heat and vaporize a solid target in a vacuum chamber. But in pulsed laser deposition, the vaporized material travels in a luminescent plume toward a heated substrate where the material is deposited. The plume is a dense plasma of energetic electrons and neutral atoms, ions, and molecules in both excited and ground states. The plume’s contents are laid down in the same composition as the starting polycrystalline target material, but in the form of a film whose crystalline structure is locked into place by the substrate’s atomic template. Although the first pulsed laser deposition experiment was conducted in 1965, the technique has been employed widely only since 1987, when it was discovered that it can be used to grow high-temperature superconducting films.
ORNL researchers have taken high-tech snapshots of many a plume on its trip from target to substrate. They have used an assortment of sophisticated tools to get the picture. These include a fast-intensified charge-coupled detector camera to acquire images of the fluorescent plasma; optical emission and absorption spectroscopies that differentiate among the plume’s atomic and molecular species and states; and an ion probe, which measures ion energy and plasma density. From these tools, scientists can learn how fast the plume is moving and how much material in different atomic forms is present in the plume’s different parts.
By monitoring the plume, ORNL researchers can predict whether the film being produced will be uniformly thick. They can determine which conditions—laser beam energy and wavelength, gas pressure, substrate temperature—have changed and how much any change should be corrected to optimize film quality. From the diagnostics, they have found that most of the plume blasts from target to substrate in 20 microseconds and that the fast plume material moves from 1 to 5 centimeters per microsecond.
How fast the material moves, where it goes, how much rebounds from the substrate, and what type of product is formed depend partly on whether the plume is in a vacuum or background gas. For example, if the target is graphite in a background gas like argon, all-carbon buckyballs can be produced. If graphite is zapped in a vacuum with a laser beam, the product could be an amorphous diamond film that emits electrons when subjected to a strong electric field. Because this film is a superhard, transparent field emitter which can excite phosphor coatings, one company is using laser ablation to make amorphous diamond films for flat panel displays; these devices are needed to make much less bulky television sets and computer monitors, similar to the flat screens of notebook computers. ORNL researchers are looking at ways to deposit amorphous diamond films that could emit electron beams in lieu of light to pattern silicon chips. In this way, more circuits can be packed on each chip, making a chip both smaller and smarter.
ORNL’s plume diagnostics have revealed just how different background gases and varying gas pressures slow down the plume, causing the material to cluster together. As a rule, the gas absorbs energy from collisions between the expanding plasma and gas molecules, slowing down many plasma species. As the gas pressure is increased, some of the rapidly expanding plasma escapes with few collisions, but most gets caught in a snowplowed pile of gas which acts like a shock wave. As a result, the plume splits into two or three components. ORNL researchers developed the first accurate model of plume splitting and were among the first groups to understand it.
Monitoring of many types of ablation plumes showed that clusters of bound atoms ejected from the target can sometimes ruin an amorphous diamond film, but that clusters created by collisions with a background gas—the “third component” of a split plume—can be useful in other ways. For example, in experiments involving doping zinc telluride films to learn how to make cheaper blue-light-emitting diodes, the nitrogen gas itself was incorporated in the film. When film growth was conducted using each of the three components of material observed with the plume pictures, researchers produced films that possess quite different electronic properties. The best films were obtained when the nitrogen pressure was high enough to kill the damaging superfast “first” component, but the nitrogen content was low enough to avoid formation of the clunky nanoparticles of the “third” plume component. ORNL researchers now are intentionally making larger clusters and nanoparticles at higher pressures, from supertough nanotubes to nanocrystallites (which emit light in electric fields), all of interest for flat panel displays.
In respect to laser deposition plumes, ORNL researchers don’t use film to get good pictures, but instead use pictures to get good films.
The research is supported by DOE’s Office of Energy Research, under the Division of Materials Sciences in the Office of Basic Energy Sciences.