| Research
|
Without catalysts, you wouldn't be driving a car, using a computer, or tying your shoelaces. Catalysts— materials that speed up a reaction but aren't consumed by it—are vital to refining crude oil and producing plastic for computer casings and even for manufacturing the plastic tips on your shoelaces. Unfortunately, catalysts are typically designed with a “one size fits all” mentality. At Pacific Northwest National Laboratory's Institute for Interfacial Catalysis , chemical engineer Yong Wang is taking a revolutionary approach to tailor catalysts, making them more efficient and safer. By making catalysts more effective, engineers can miniaturize systems. Wang and his research team develop a catalyst for a desired reaction first. Then they engineer it onto an innovative support structure. Finally, they design the application around the supported catalyst. Wang pioneered this approach when the team developed a catalyst that transforms hydrocarbon and steam into hydrogen and oxygen in milliseconds, rather than seconds. The result is a catalytic fuel processing reactor system that is smaller than a dime. “Technologies such as this have the potential to transform the way we live and to reduce our dependence on imported oil,” Wang said. “We are looking at more efficient ways to convert natural gas to gasoline and diesels. We are also looking at generating hydrogen—a clean and efficient source of power—from both fossil and renewable sources.” His work has led to 60 patents, with 30 more patents pending, plus the formation of a company that is one of the leading developers of microchannel technology systems . Recently named the 2006 Asian American Engineer of the Year Award by Chinese Institute of Engineers-USA, Wang has also earned two R&D 100 awards and the Presidential Green Chemistry Award.Submitted by DOE's Pacific Northwest National Laboratory |
||||||||||||||||||||||
|
Check out symmetry—the
|
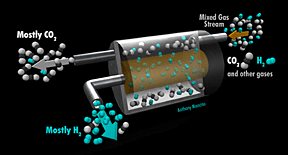
Collaboration Aims to Reduce Greenhouse Gas Emissions
While alternative energy sources will gradually increase their contribution to the world's energy supply, most projections agree that conventional fossil energy generation will continue to dominate for at least the next 15 years. U.S. energy demand alone currently results in billions of tons of carbon dioxide emissions annually creating a variety of environmental challenges. A new and economical technology for the separation and capture of carbon dioxide from fossil fuel conversion processes could lead to a significant reduction in greenhouse-gas emissions. Submitted by DOE's Los Alamos National Laboratory |