Research
Highlights...

|
PPPL's
Jon Menard
|
|
 |
Number 247 |
November 05, 2007 |
|
Chemical force microscopy probes nanotubes
|
|
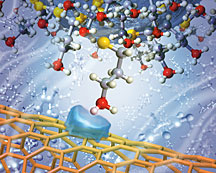
An artist's representation of an amine functional group attached to an AFM tip approaching a carbon nanotube surface in toluene solution. Translucent blue shape on the nanotube represents the polarization charge forming on the nanotube as the result of the interaction with the approaching molecule. Chemical force microscopy measures the tiny forces generated by this single functional group interaction. (Illustration by Scott Dougherty, LLNL) |
Researchers for the first time have been able to measure a specific interaction for a single functional group with carbon nanotubes using chemical force microscopy—a nanoscale technique that measures interaction forces using tiny spring-like sensors. Functional groups are the smallest specific group of atoms within a molecule that determine the characteristic chemical reactions of that molecule. A recent report by a team of researchers and colleagues at DOE's Lawrence Livermore National Laboratory found that the interaction strength does not follow conventional trends of increasing polarity or repelling water. Instead, it depends on the intricate electronic interactions between the nanotube and the functional group. “This work pushes chemical force microscopy into a new territory,” said Aleksandr Noy, lead author of the paper that appears in the Oct. 14 online issue of the journal, Nature Nanotechnology.
[Anne Stark, 9 25/422-9799,
stark8@llnl.gov] |
|
NETL scientist on methane hydrates research cruise
Scientist Kelly Rose from DOE's National Energy Technology Laboratory is participating as lead sedimentologist on a South Korean methane hydrates expedition in the Ulleung Basin in Korea 's East Sea. This is the latest in a series of major gas hydrate field projects to which Rose has provided geologic expertise in the past 18 months. Prior expeditions have taken her to the Bay of Bengal, the North Slope of Alaska, and the South China Sea. Rose's primary interest is to study the nature of the gas hydrate-bearing sediments to investigate the relationships between lithology, sedimentary structure and other geological features on gas hydrate occurrence.
[Linda Morton, 304/285-4543,
Linda.morton@netl.doe.gov] |
|
NREL reports on Clean Cities savings
Clean Cities coalitions around the nation displaced the equivalent of 375 million gallons of gasoline in 2006, according to a recent report from DOE's National Renewable Energy Laboratory. The amount of gasoline displaced in 2006 was 50 percent more than the 250 million gallons in 2005. Clean Cities coalitions are on track to reach 3.2 billion gallons of gasoline displaced in 2020, exceeding their established goal by 700 million gallons. The study was compiled from voluntary reports that represent a subset of the activities going on throughout the nation and indicates the impact of the coalitions and their priorities.
[Sarah Holmes Barba, 303/275-3023,
sarah_barba@nrel.gov] |
|
Molecular Foundry receives LEED gold certification
|
|
Berkley's Molecular Foundry |
The Molecular Foundry ,a nanotechnology research facility located at Lawrence Berkeley National Laboratory, has received a U.S. Green Building Council Leadership in Energy and Environmental Design (LEED) gold certification. The LEED green building rating system is the benchmark for the design, construction, and operation of energy efficient buildings. The Molecular Foundry's gold rating — the second-highest ranking obtainable under the system — is based on the utilization of a myriad of features, including optimally designed electrical and HVAC systems, an energy-efficient chiller and boiler plant, and the innovative design of traditionally energy-intensive areas such as labs, a cleanroom, and a server room. The Molecular Foundry consumes 28 percent less energy than the already-stringent California building efficiency standard. It is one of only three Office of Science facilities to receive LEED gold certification, and is one of five Office of Science Nanoscale Science Research Centers.
[Dan Krotz, 510/486-6641,
dakrotz@lbl.gov]
|
|
|
Menard Named NSTX
Program Director
 |
Jon Menard |
As the new Program Director for the National Spherical Torus Experiment (NSTX) at DOE's Princeton Plasma Physics Laboratory (PPPL), Jon Menard is spending the majority of his time helping to define the scientific research program for the fusion energy experiment. NSTX magnetically confines a hot ionized gas, or plasma, shaped like a sphere with a hole through its center—a spherical torus (ST). This differs from the conventional donut-shaped plasma used as the fuel for the production of fusion energy. The smaller ST configuration could lead to the development of more compact, economical fusion reactors.
In his new post, Menard works closely with NSTX Project Director Masa Ono, whose role will remain working with engineers, technicians, and the national team of physicists to implement the facility capabilities and diagnostics that make the research program possible. “Clearly, the Program and Project Directors must work very closely together for NSTX to remain successful,” Menard noted.
Menard joined the research staff at PPPL in 1999 after conducting post-doctoral research at the Laboratory. He received a bachelor's degree in nuclear engineering from the University of Wisconsin-Madison in 1992, and a master's and a Ph.D. in plasma physics from Princeton University in 1994 and 1998, respectively. Among his honors, Menard received the 2006 Kaul Prize for Excellence in Plasma Physics Research and Technology Development and the 2002 Presidential Early Career Award for Scientists and Engineers.
Menard's first task is to organize a five-year plan for the NSTX research program. He is also working on physics design activities for a proposed next-step ST device, the National High-power Advanced Torus Experiment (NHTX). In addition, Menard is focusing on how NSTX results can contribute strongly to ITER, an unprecedented international fusion experiment that will be built in Cadarache, France and operate in 2016.
Menard described his new job as “a challenge I look forward to. I've worked with many members of the NSTX team from the research side. I really enjoy doing research, but I also increasingly appreciate the value of helping to create a productive research environment — an interesting coupling.”
Submitted by DOE's
Princeton Plasma Physics Laboratory
|
|