-
 Led by Alex Zettl of the Materials Sciences Division, researchers at DOE’s Lawrence Berkeley National Laboratory have used the world’s most powerful transmission electron microscope, TEAM 0.5 at the National Center for Electron Microscopy, to make the first real-time movie of individual carbon atoms in motion. The atoms are seen repositioning themselves around the edge of a hole punched in a graphene sheet by the microscope’s focused electron beam.
Led by Alex Zettl of the Materials Sciences Division, researchers at DOE’s Lawrence Berkeley National Laboratory have used the world’s most powerful transmission electron microscope, TEAM 0.5 at the National Center for Electron Microscopy, to make the first real-time movie of individual carbon atoms in motion. The atoms are seen repositioning themselves around the edge of a hole punched in a graphene sheet by the microscope’s focused electron beam.
Full Story
-
 Scientists at DOE's Los Alamos National Laboratory have produced the world's first Map of Science—a high-resolution graphic depiction of the virtual trails scientists leave behind when they retrieve information from online services. Bollen and colleagues from LANL and the Santa Fe Institute collected usage-log data gathered from a variety of publishers, aggregators, and universities from 2006 to 2008, nearly 1 billion online information requests.
Scientists at DOE's Los Alamos National Laboratory have produced the world's first Map of Science—a high-resolution graphic depiction of the virtual trails scientists leave behind when they retrieve information from online services. Bollen and colleagues from LANL and the Santa Fe Institute collected usage-log data gathered from a variety of publishers, aggregators, and universities from 2006 to 2008, nearly 1 billion online information requests.
Full Story
-
 A team of scientists at DOE's Lawrence Livermore National Laboratory has developed a technology that filters out silica from geothermal waters, allowing geothermal electrical plants to work more efficiently and to market a byproduct, silica. While the United States leads the world in geothermal electrical production, one problem in geothermal turbine facilities is that silica clogs the pipes, filters and heat exchangers.
A team of scientists at DOE's Lawrence Livermore National Laboratory has developed a technology that filters out silica from geothermal waters, allowing geothermal electrical plants to work more efficiently and to market a byproduct, silica. While the United States leads the world in geothermal electrical production, one problem in geothermal turbine facilities is that silica clogs the pipes, filters and heat exchangers.
Full Story
-
 DOE's National Energy Technology Laboratory and the University of Pittsburgh have simulated methane hydrate decomposition at the molecular level under conditions typical of those anticipated in methane production scenarios. Researchers observed transitory, partial-hydrate structures at the decomposing hydrate interface.
DOE's National Energy Technology Laboratory and the University of Pittsburgh have simulated methane hydrate decomposition at the molecular level under conditions typical of those anticipated in methane production scenarios. Researchers observed transitory, partial-hydrate structures at the decomposing hydrate interface.
Full Story
-
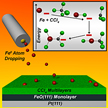 Previously thought to be a simple reaction, experimental scientists and theoreticians at DOE’s Pacific Northwest National Laboratory found that tiny iron particles instigate a complex series of reactions that turn carbon tetrachloride into harmless chemicals. Using "atom dropping" experiments at DOE’s EMSL, a delicate and time-consuming process, the experimental team reacted iron clusters and carbon tetrachloride under ideal conditions, measuring the dependence of products on cluster size, concentration, and temperature.
Previously thought to be a simple reaction, experimental scientists and theoreticians at DOE’s Pacific Northwest National Laboratory found that tiny iron particles instigate a complex series of reactions that turn carbon tetrachloride into harmless chemicals. Using "atom dropping" experiments at DOE’s EMSL, a delicate and time-consuming process, the experimental team reacted iron clusters and carbon tetrachloride under ideal conditions, measuring the dependence of products on cluster size, concentration, and temperature.
Full Story
 Led by Alex Zettl of the Materials Sciences Division, researchers at DOE’s Lawrence Berkeley National Laboratory have used the world’s most powerful transmission electron microscope, TEAM 0.5 at the National Center for Electron Microscopy, to make the first real-time movie of individual carbon atoms in motion. The atoms are seen repositioning themselves around the edge of a hole punched in a graphene sheet by the microscope’s focused electron beam.
Led by Alex Zettl of the Materials Sciences Division, researchers at DOE’s Lawrence Berkeley National Laboratory have used the world’s most powerful transmission electron microscope, TEAM 0.5 at the National Center for Electron Microscopy, to make the first real-time movie of individual carbon atoms in motion. The atoms are seen repositioning themselves around the edge of a hole punched in a graphene sheet by the microscope’s focused electron beam. Scientists at DOE's Los Alamos National Laboratory have produced the world's first Map of Science—a high-resolution graphic depiction of the virtual trails scientists leave behind when they retrieve information from online services. Bollen and colleagues from LANL and the Santa Fe Institute collected usage-log data gathered from a variety of publishers, aggregators, and universities from 2006 to 2008, nearly 1 billion online information requests.
Scientists at DOE's Los Alamos National Laboratory have produced the world's first Map of Science—a high-resolution graphic depiction of the virtual trails scientists leave behind when they retrieve information from online services. Bollen and colleagues from LANL and the Santa Fe Institute collected usage-log data gathered from a variety of publishers, aggregators, and universities from 2006 to 2008, nearly 1 billion online information requests. A team of scientists at DOE's Lawrence Livermore National Laboratory has developed a technology that filters out silica from geothermal waters, allowing geothermal electrical plants to work more efficiently and to market a byproduct, silica. While the United States leads the world in geothermal electrical production, one problem in geothermal turbine facilities is that silica clogs the pipes, filters and heat exchangers.
A team of scientists at DOE's Lawrence Livermore National Laboratory has developed a technology that filters out silica from geothermal waters, allowing geothermal electrical plants to work more efficiently and to market a byproduct, silica. While the United States leads the world in geothermal electrical production, one problem in geothermal turbine facilities is that silica clogs the pipes, filters and heat exchangers. DOE's National Energy Technology Laboratory and the University of Pittsburgh have simulated methane hydrate decomposition at the molecular level under conditions typical of those anticipated in methane production scenarios. Researchers observed transitory, partial-hydrate structures at the decomposing hydrate interface.
DOE's National Energy Technology Laboratory and the University of Pittsburgh have simulated methane hydrate decomposition at the molecular level under conditions typical of those anticipated in methane production scenarios. Researchers observed transitory, partial-hydrate structures at the decomposing hydrate interface.  Previously thought to be a simple reaction, experimental scientists and theoreticians at DOE’s Pacific Northwest National Laboratory found that tiny iron particles instigate a complex series of reactions that turn carbon tetrachloride into harmless chemicals. Using "atom dropping" experiments at DOE’s EMSL, a delicate and time-consuming process, the experimental team reacted iron clusters and carbon tetrachloride under ideal conditions, measuring the dependence of products on cluster size, concentration, and temperature.
Previously thought to be a simple reaction, experimental scientists and theoreticians at DOE’s Pacific Northwest National Laboratory found that tiny iron particles instigate a complex series of reactions that turn carbon tetrachloride into harmless chemicals. Using "atom dropping" experiments at DOE’s EMSL, a delicate and time-consuming process, the experimental team reacted iron clusters and carbon tetrachloride under ideal conditions, measuring the dependence of products on cluster size, concentration, and temperature.  Small interactions pave way to big solutions
Small interactions pave way to big solutions Largest-ever LNG fire test probes hazards from tanker spills
Largest-ever LNG fire test probes hazards from tanker spills