- Number 301 |
- December 7, 2009
Simulation, calculations show hydroxide ions orientation in water
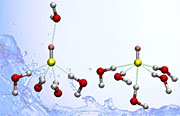
By studying hydroxide ions in
water, the team found that the ion
(center) can form two different
structures: one with five hydrogen
bonds
(left) and the other with
four.
Whole water molecules form complex shapes around hydroxide ions, simple negatively charged particles, according to scientists at DOE's Pacific Northwest National Laboratory. The shapes are the result of hydrogen bonds between the ions and the molecules. This research answers the question, debated in scientific circles for more than 70 years, of how hydroxide ions get oriented in water. Knowing how hydroxide ions are arranged in water could aid scientists in fine-tuning current industrial processes, such as manufacturing biodiesel, making processes more efficient or less wasteful. Further, it could assist in developing future industrial processes, such as turning poplar trees and other vegetation into automotive fuel. This work, done using NWChem at DOE’s EMSL, graced the cover of Chemical Physics Letters.
[Kristin Manke, 509.372.6011,
kristin.manke@pnl.gov]
