- Number 304 |
- February 1, 2010
Finding the perfect spot to rest
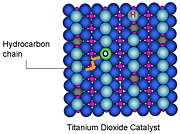
Like spokes on a gear, alkyl
chains twist and stop at specific
spots on the surface of the
catalyst titanium dioxide.
Prima donnas. Floppy hydrocarbon chains are quite particular about where they’ll rest on a catalyst, according to a recent study by the Department of Energy’s Pacific Northwest National Laboratory and the University of Texas at Austin. On the surface of the common catalyst titanium dioxide, these hydrocarbon chains settle into valleys of titanium atoms, avoiding nearby ridges of oxygen atoms. The alkyl chains are drawn to these locations because of a weak attraction to the titanium. Understanding where the alkyl chains reside aids scientists in precisely modifying catalyzed reactions, such as the those used in producing biofuels. This work was done using instruments at the Department of Energy’s EMSL, a national scientific user facility at PNNL.
[Kristin Manke, 509.372.6011,
kristin.manke@pnl.gov]
