- Number 368 |
- July 30, 2012
Ionic liquid improves speed and efficiency of hydrogen-producing catalyst
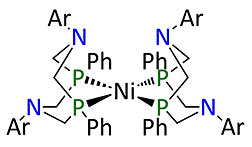
Combined with an acidic ionic liquid, this
catalyst can make hydrogen gas fast and
efficiently.
The design of a nature-inspired material that can make energy-storing hydrogen gas has gone holistic. Usually, tweaking the design of this particular catalyst—a work in progress for cheaper, better fuel cells—results in either faster or more energy-efficient production but not both. Now, researchers at DOE’s Pacific Northwest National Laboratory have taken a holistic approach that allows the creation of hydrogen faster without a loss in efficiency.
This new approach requires the entire system—the hydrogen-producing catalyst and the liquid environment in which it works—to overcome the speed-efficiency tradeoff. The results, in the Proceedings of the National Academy of Sciences, provide insights into making better materials for energy production.
“Our work shows that the liquid medium can improve the catalyst's performance,” said chemist Dr. John Roberts of the Center for Molecular Electrocatalysis at PNNL. “It's an important step in the transformation of laboratory results into useable technology.”
The results also provide molecular details into how the catalytic material converts electrical energy into the chemical bonds between hydrogen atoms. This information will help the researchers build better catalysts, ones that are both fast and efficient, and made with the common metal nickel instead of expensive platinum.[Kristin Manke, 509.372.6011,
kristin.manke@pnnl.gov]
