- Number 377 |
- December 3, 2012
Scientists capture lithium-ion batteries in nanoscale action
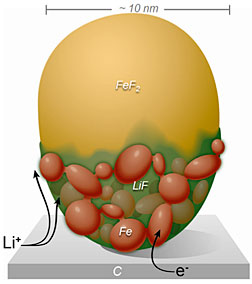
This diagram shows the spread of positively
charged lithium ions across the custom-built
FeF2 nanoparticle. The conversion reaction
swept rapidly across the surface before
proceeding more slowly through the bulk
of the particle.
The cherished portability of many popular electronics, from smart phones to laptops, mostly comes courtesy of lithium-ion batteries. Unfortunately, these dense and lightweight energy storage devices degrade over time, steadily losing total capacity even when sitting idle on the shelf.
Now, researchers at Brookhaven National Laboratory and collaborating institutions have developed methods of examining lithium-ion reactions in real-time with nanoscale (billionths of a meter) precision, offering unprecedented insights into these crucial materials. The technique uses a novel electrochemical cell and transmission electron microscopy (TEM) to track lithium conversion and precisely expose subtle changes that occur in batteries’ electrodes over time.
“We’ve opened a fundamentally new window into this popular technology,” said Brookhaven Lab physicist and lead author Feng Wang. “The live, nanoscale imaging may help pave the way for developing longer-lasting, higher-capacity lithium-ion batteries. That means better consumer electronics, and the potential for large-scale, emission-free energy storage.”
Scientists at the Lab’s Center for Functional Nanomaterials observed the lithium conversion process as it unfolded across iron fluoride (FeF2) nanoparticles. These real-time experimental observations, supported by advanced computation, revealed that the lithium ions swept rapidly across the surface of the nanoparticles in a matter of seconds before moving slowly through the bulk in a layer-by-layer fashion.
“Although many questions remain about the true mechanisms behind this conversion reaction, we now have a much more detailed understanding of electron and lithium transport in lithium-ion batteries,” said Brookhaven physicist and study coauthor Jason Graetz.[Justin Eure, 631.344.2347,
jeure@bnl.gov]
