- Number 385 |
- April 1, 2013
Computational study of ionic liquids illuminates detailed CO2 interactions
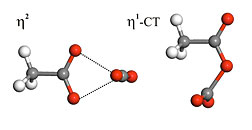
The η2 and η1-CT structures of the
acetate-CO2 complex..
Ionic liquids (ILs), which can be thought of as salts that are molten at room temperature, are being studied for use as part of CO2 adsorption and/or separation technologies. These applications depend on having strong interactions between the CO2 and the ions of the IL. In order for significant advances to occur in this area of research, the interaction between the CO2 and each IL must be understood and described with accuracy. Computational methods are used to describe these interactions on a molecular level.
National Energy Technology Laboratory scientist Jan Steckel has used a variety of methods to elucidate the complex nature of the interactions between CO2 and acetate ion. The results of this study were published recently in the Journal of Physical Chemistry A. The acetate ion was chosen because it is representative of the anions used in many ILs currently under investigation as CO2 sorbents or as part of a separation technology.
Dr. Steckel has shown that the acetate-CO2 potential energy surface is very complex. Eight energy minima, representing the most stable configurations, were located and characterized using computational methods that apply first-principles molecular orbital calculations to obtain an accurate description of these interactions at the molecular level.
The most stable structure is denoted η2, (eta 2) where the CO2 interacts with both oxygen atoms of the acetate. This complex structure is predicted to have a binding energy of -10.6 kcal/mol, a measure of stability. There are several other complexes with binding energies close to -8.5 kcal/mol, but of these, the η1-CT complex (eta 1) is unique. This complex is notable because the CO2 is bent to about 140°, the C atoms of the CO2 are only 1.54 Å away from the O of acetate, and there is evidence of charge being transferred from the acetate to the CO2 upon complexation.Using these interaction energies as benchmarks, it was possible to investigate the degree to which more affordable methods can describe these complexes. Unfortunately, many popular and affordable computational methods do not succeed in describing the η1-CT complex accurately. This study helps to provide a clear understanding of the acetate-CO2 interaction and supplies previously missing energetic and structural benchmark data. However, another important contribution made by this work is the revelation that widely-used but less accurate methods fail to accurately describe this interaction.
[Linda Morton, 304.285.4543,
Linda.morton@netl.doe.gov]
