- Number 387 |
- April 29, 2013
The nanostructure of edible fats
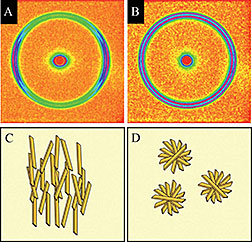
X-ray diffraction patterns reveal the
orientation of fat crystals. The distribution
and directionality of these crystal
nanostructures (parallel to the shear field
in C, randomly arranged in D) affects the
flavor and texture of foods.
Researchers at DOE's Brookhaven are using the National Synchrotron Light Source (NSLS) to categorize the many facets of fat crystals. They’ve learned that the distribution and directionality of these crystal nanostructures affects the flavor and texture of foods.
From butter in croissants to cocoa solids in chocolate, edible fats pack a flavor punch that delights like no other macronutrient we consume. Fats are the most energy dense macronutrients, providing more than twice as many kilocalories per gram as proteins or carbohydrates, which may be the reason we’ve developed a taste for them. Fats are an efficient method of fueling a surviving species, but what gives them their oh-so-delicious disposition?
As explained in a review paper by NSLS user Alejandro Marangoni, published in Soft Matter, fats are made up of fractal-like crystalline structures, which give rise to properties such as flavor, texture, meltability, and mouthfeel. For example, six different forms of crystal structure have been identified for cocoa butter. But only one form will turn out chocolate that tastes and feels good to eat.
Marangoni and his collaborators used x-ray diffraction at NSLS to study their complex arrangements.
“We can witness a very diverse assortment of crystal habits – spherulites, needle-like crystals, microplatelets, disordered crystal aggregates, spherical crystal aggregates, fractal-like aggregates, and even some morphologies that defy proper description.
Despite the wide variety of crystal morphologies, they all share some common attributes: (1) the structures are awe-inspiringly beautiful when viewed under a polarized light microscope and (2) the crystalline mass in a network of these crystals is distributed in a fractal fashion.”
The discovery and characterization of these nanoplatelets adds to the knowledge upon which the food industry bases its design and engineering of food materials. Understanding how the physical chemistry of fats affects the way foods taste and feel could also potentially be useful when trying to curb the excessive consumption of fat-rich foods.
[Chelsea Whyte, 631.344.8671,
cwhyte@bnl.gov]
