- Number 389 |
- May 27, 2013
Would you hire this catalyst?
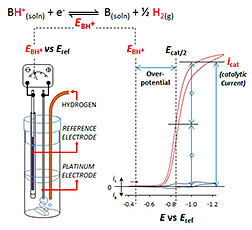
Researchers use an electrochemical cell to
determine the overpotential or energy
efficiency of a catalyst reaction that converts
electrical energy into chemical energy,
specifically the bond between two hydrogen
atoms. This work was done at the Center for
Molecular Electrocatalysis, an Energy Frontier
Research Center funded by DOE's Office of
Basic Energy Sciences. If you want to learn
more about research at the Center for
Molecular Electrocatalysis, follow us on
Twitter @CME_EFRC.
A catalyst that quickly produces chemical fuel from wind energy but uses more energy than it stores won't get the job; however, scientists didn't have a way to measure the energy wasted in certain liquids, until DOE's Pacific Northwest National Laboratory devised a quick, elegant technique. This method, by Dr. John Roberts and Dr. R. Morris Bullock, was published in Inorganic Chemistry.
"We could make some educated guesses and do back-of-the-envelope calculations as to over potential in organic solvents, but it wasn't good enough," said Bullock, Director of the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by DOE's Office of Basic Energy Sciences.
Their method begins with a pair of ordinary electrodes, which act as a "volt meter," to measure how energetic the electrons need to be in the ideal case of converting electrons or electrical energy into a bond between two hydrogen atoms. Next, the ideal voltage is compared to the voltage required for a real catalyst. In the study, the researchers used a nickel diphosphine complex, developed by Bullock and his colleagues at the Center for Molecular Electrocatalysis, as the catalyst. The difference in these two voltages is the first accurate measure of the overpotential for any catalyst operating in acetonitrile.
Roberts and Bullock demonstrated the technique with the reaction in fluorobenzene and organic solvent/water mixtures as well. "The energy efficiencies of catalyst systems interconverting electrical energy with hydrogen fuel may now be compared on an equal footing, using any solvent," said Bullock.
The team ran the experiments with different acids. In every case, the results agreed perfectly with thermodynamic calculations.
"This research allows us to compare energy efficiencies as a function of solvent," said Roberts, an inorganic chemist who led this study and conducted the electrochemical experiments. "Changing the solvent can make the catalyst both faster and more energy efficient. This happens because rate and energy efficiency are related to proton movement, and proton movement is related to the solvent, the catalyst, and how they interact."
The research was funded by the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by DOE, Office of Science, Office of Basic Energy Sciences.[Kristin Manke, 509.372.6011,
kristin.manke@pnnl.gov]
