- Number 391 |
- June 24, 2013
NETL studies unique properties of quantum alloys
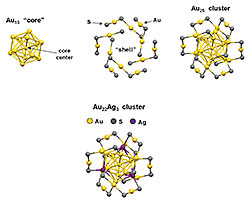
Structure of a Quantum Alloy: The top
illustrations depict how 13 Au atoms forming
an icosohedron “core” bond with sulfur and
gold atoms in a “shell” to form the Au25
catalyst for CO2 remediation. The bottom
illustration shows how 3 of the Au atoms in
the shell can be replaced with Ag to form
the Au22Ag3 quantum alloy.
When common household metals, such as copper, gold, or silver, are reduced in size to clusters that consist of a few dozen atoms, the materials develop completely unexpected properties. One example occurs for gold, which is inert in its bulk form, but which develops the ability to efficiently catalyze chemical reactions when made as atomic-scale clusters that are over 1,000 times smaller than a human hair and invisible to the eye. These properties result from quantum confinement effects, a term scientists use to describe how small clusters and particles evolve with size. Quantum effects are the principal driving force behind the field of nanotechnology, where engineers manipulate the colors, electrical conductivity, and chemistry of matter simply by controlling and constraining size at the atomic scale.
Researchers at DOE's National Energy Technology Laboratory have been using quantum effects to design radical new catalysts capable of converting CO2 emissions into fuels, chemicals, and plastics. Their unique discovery involves shrinking gold into a system consisting of just 25 atoms, commonly referred to as Au25. This material was recently demonstrated by NETL to be one of the most efficient catalysts ever reported for applications involving the use of carbon dioxide (CO2). In order to further improve catalytic activity and reduce production costs, NETL has been investigating ways to replace some of the Au (gold) atoms with Ag (silver), Cu (copper), and other metals. The mixing of metals to improve properties is commonly called alloying, but these small clusters behave very differently than bulk systems, and have been named quantum alloys to highlight their unique properties.
When researchers replace just 3 atoms in Au25 with Ag or Cu, the catalysts take on exciting new properties. Laboratory experiments show that forming quantum alloys completely changes how the atoms in these clusters bond to each other, how they absorb light (e.g., their color), and more importantly, how they catalyze chemical reactions involving CO2.
Researchers have also used sophisticated computational modeling to probe the properties of these clusters. These calculations helped to predict where the Ag and Cu atoms prefer to reside in the alloy and how common gases, such as CO2 and oxygen, interact with the alloy.
Future work in this area will continue to address how to form these alloys and evaluate their ability to effectively convert CO2 into value-added products. These results were recently published in the highly regarded Journal of Physical Chemistry C (2013, 117, 7914-7923).[Linda Morton, 304.285.4543,
Linda.morton@netl.doe.gov]
