- Number 403 |
- December 16, 2013
Inside look at a MOF in action
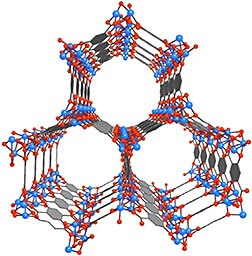
Mg-MOF-74 is an open metal site MOF whose
porous crystalline structure could enable it
to serve as a storage vessel for capturing
and containing the carbon dioxide emitted
from coal-burning power plants.
A unique inside look at the electronic structure of a highly touted metal-organic framework (MOF) as it is adsorbing carbon dioxide gas should help in the design of new and improved MOFs for carbon capture and storage. Researchers at DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab) have recorded the first in situ electronic structure observations of the adsorption of carbon dioxide inside Mg-MOF-74, an open metal site MOF that has emerged as one of the most promising strategies for capturing and storing greenhouse gases.
MOFs are molecular systems consisting of a metal oxide center surrounded by organic molecules that form a highly porous three-dimensional crystal framework with a sponge-like capacity for adsorbing greenhouse gases. In a study supported by Berkeley’s Energy Frontier Research Center, the team used Near Edge X-ray Absorption Fine Structure (NEXAFS) at Berkeley Lab’s Advanced Light Source (ALS) to obtain what are believed to be the first ever measurements of chemical and electronic signatures inside of a MOF during gas adsorption.
Demonstrating that NEXAFS spectroscopy is an effective tool for the study of MOFs and gas adsorption, these measurements revealed that open metal site MOFs have significant X-ray spectral signatures highly sensitive to the adsorption of carbon dioxide and other molecules. Calling upon the resources of the National Energy Research Scientific Computing Center and the Molecular Foundry, the research team provided fundamental insights into why some MOFs work better than others, which, in turn, should help scientists predict the best metals to use in the design of future MOFs. The research was led by Jeff Kortright of Berkeley Lab’s Materials Sciences Division.
[Lynn Yarris, 510.486.5375,
lcyarris@lbl.gov]
