- Number 420 |
- August 18, 2014
Researchers use waste slag to create energy and cut emissions
Researchers use waste slag to create
energy and cut emissions.
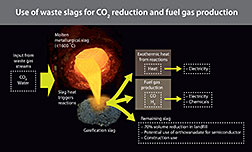
Slag is a molten mixture of process waste ashes from the power and metallurgical industries. In gasification, slag is made from mineral impurities that remain after a carbon feedstock such as coal has been gasified. In metal refining, slag contains impurities removed from a metal while it is refined. Slag is basically waste that is landfilled in many countries, including the U.S. Two researchers at DOE's National Energy Technology Laboratory have found a way to make slag more valuable. They determined that by mixing two particular types of slag at a unique ratio they can generate energy and fuels.
One of the slags is a byproduct of a metallurgical process that is rich in calcium oxide. The other slag contains high levels of vanadium (III) oxide generated during gasification using petroleum coke-based carbon feedstocks. When the two slags, molten at discharge, are mixed together in the presence of carbon dioxide (CO2), the resulting chemical reactions give off a very large amount of heat—enough to turn a turbine and generate electricity. Simultaneously, the same chemical reactions change CO2 into carbon monoxide (CO), which can be combusted with oxygen for additional power or used as a raw material for producing chemicals such as synthetic petroleum.
Hydrogen gas, which is useful in chemical manufacturing and industrial applications including fuel cells, can be made from water using the same procedure. The reactions that yield these products are described in an article recently published in the International Journal of Hydrogen Energy.
The most important aspect of the two slag interactions is that, by mixing them while molten in the presence of CO2, the potential exists to reduce the quantity of CO2 entering the atmosphere by converting it into CO, which can be used in a power plant as fuel or by the chemical industry as a raw material, reducing the amount of carbon needed in these processes. That is the real objective of this work—reducing our CO2 emissions.[Linda Morton, 304.285.4543,
Linda.morton@netl.doe.gov]
