- Number 443 |
- July 13, 2015
X-Rays and electrons map catalytic reactions in real time
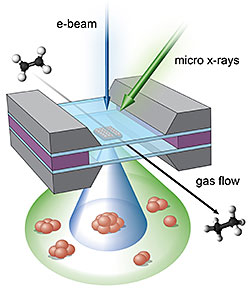
Illustration of the micro-reactor showing
the complementary imaging areas of the
electron beams (blue) and x-rays (green),
which combine to reveal a full portrait of
the real-time reaction.
A new technique pioneered at Brookhaven Lab that combines electron microscopy and synchrotron x-rays reveals atomic-scale changes during catalytic reactions in real time and under real operating conditions. The work makes use of a newly developed micro-reactor the size of a mosquito to combine x-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM) at Brookhaven’s National Synchrotron Light Source (NSLS) and Center for Functional Nanomaterials (CFN), respectively. The results demonstrate a powerful operando technique—from the Latin for "in working condition"—that may revolutionize research on catalysts, batteries, fuel cells, and other major energy technologies.
"We tracked the dynamic transformations of a working catalyst, including single atoms and larger structures, during an active reaction at room temperature," said CFN scientist Eric Stach. "This gives us unparalleled insight into nanoparticle structure and would be impossible to achieve without combining two complementary operando techniques."
To prove the efficacy of the micro-reactor, the scientists tracked the performance of a platinum catalyst during the conversion of ethylene to ethane, a model reaction relevant to many industrial synthesis processes.
"With TEM, we take high-resolution pictures of the particles to directly see their size and distribution," said Stach. XAS complements TEM by identifying the sample’s chemical composition—in this instance, the distribution of platinum particles. Further, data from the two techniques can be combined to deduce precise sub-nanometer features that would otherwise be hidden from TEM.
"The XAS and TEM data, analyzed together, let us calculate the numbers and average sizes of not one, but several different types of catalysts," said coauthor and Yeshiva University scientist Anatoly Frenkel, who led the x-ray experiments. "Running the tests in an operando condition lets us track broad changes over time, and only the combination of techniques could reveal all catalytic particles."
Said study coauthor Ralph Nuzzo of the University of Illinois at Urbana-Champaign, "The size, shape, and distribution of catalysts affect their efficiency and durability. Now that we can track those parameters throughout the reaction sequence, we can better determine the ideal design of future catalysts—especially those that drive energy-efficient reactions without using expensive and rare materials like platinum."
The collaboration has already extended this operando micro-reactor approach to incorporate additional techniques—infrared and Raman spectroscopy—and plans to introduce other complex and complementary x-ray and electron probe techniques over time. Brookhaven’s brand new National Synchrotron Light Source II, with x-ray beams 10,000 times brighter than its predecessor facility, will dramatically increase the time resolution of these experiments and allow scientists to track changes in an even more dynamic fashion.
Link to article: http://www.bnl.gov/newsroom/news.php?a=11739.[Justin Eure, 631.344.2347,
jeure@bnl.gov]
