- Number 446 |
- August 24, 2015
Stress strategies for sustainable fuels
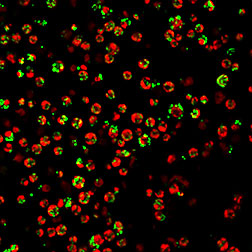
Algal cells of Chlamydomonas reinhardtii
grown under nitrogen starvation conditions
to produce lipids. The red is the
autofluorescence from the chlorophyll of
the cells while the green indicates the
lipid bodies following lipid staining with
Lipidtox Green.
(Image prepared by Rita Kuo, DOE JGI.)
Some algae like Chlamydomonas reinhardtii (or “Chlamy,” as it’s known to its large research community) produce energy-dense oils or lipids when stressed, and these lipids can then be converted into fuels. However, bioenergy researchers walk a fine line in stressing the algae just enough to produce lipids, but not enough to kill them. Published ahead online July 27, 2015 in the journal Nature Plants, a team led by scientists from DOE's Joint Genome Institute (DOE JGI), a DOE Office of Science User Facility, analyzed the genes that are being activated during algal lipid production, and in particular the molecular machinery that orchestrates these gene activities inside the cell when it produces lipids.
“We know how to stress the algae,” said the study’s first author Chew Yee Ngan of the DOE JGI. “What we don’t know is how to keep the algae alive at the same time, until now.”
Until now, very little has been known about the protein factor that can regulate lipid production. To find more of them, the team cultured Chlamy cells and starved them of nitrogen or sulfur, both of which are stress conditions to which Chlamy responds by producing lipids. They then analyzed the complex of DNA and proteins known as chromatin that define what genes are being activated, as well as the expression profiles or transcriptome, and compared these to non-stressed Chlamy cells.
“The study … demonstrated how cells can be tricked into producing lots of lipid without dying of starvation by overexpression of [the transcription factor] PSR1, which is a strategy that could potentially be applied in other industrial algal species better suited for large-scale biofuel production,” said study co-author Axel Visel, DOE JGI Deputy for Science Programs. Read the full story at http://jgi.doe.gov/keeping-algae-from-stressing-out/.[Massie Ballon, 925.927.2541,
mlballon@lbl.gov]
