|
Number 111, June 2009 |
 As predicted
As predicted
Molecular design package helps craft strongest synthetic sulfate binder
|
|
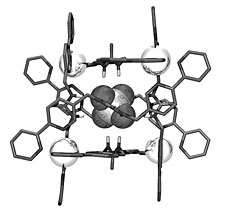
The crystal structure of sulfate was within the self-assembled cage, confirming the HostDesigner model prediction. |
A team of Chemical Sciences Division researchers, guided by a
homegrown software package, has successfully fabricated a molecular cage capable of the difficult task of sequestering sulfate in water.
In fact, the cage that formed under the prodding of Radu Custelcean and Ben Hay is the strongest synthetic sulfate binder known. This is the first example in which a computer-aided approach was used to design a cage with organized binding sites in the cavity.
“Our interest in sulfate was motivated by the specific need for technologies to remove this anion from nuclear waste,” Radu says. It is a potential solution to vitrification issues at the Hanford site stemming from the low solubility of sulfate in borosilicate glass.”
Ben and Radu, members of the Chemical Separations group have a current research goal to understand the principles of anion recognition. Negatively charged species, such as chloride, nitrate or sulfate, have distinct shapes—round, flat or, in a sulfate’s case, tetrahedral. One of the group’s objectives is to devise binding cavities that can recognize these different shapes, thereby providing the basis for selective anion separation.
Ben’s modeling showed that six urea molecules could hydrogen-bond to sulfate, yielding a complex with tetrahedral symmetry. The team reasoned that a self-assembled cage could give this arrangement if each urea molecule were the edge of a tetrahedron held together by nickel bipyridine vertices.
Starting with this as a basis for the design, the challenge was to identify linkages between the urea and bipyridine groups that would favor the desired arrangement. This is where Ben’s computer-aided molecular design software, called HostDesigner, comes in.
Using a virtual library of hydrocarbon fragments, HostDesigner within minutes generates and evaluates large numbers of possible linkages on a desktop computer. After evaluating more than a quarter million structures, HostDesigner presented Radu and Ben with a list of the most promising cage components. Molecular modeling allowed them to pare these candidates down to a small number of feasible synthetic targets.
One of the “designer” cage components was synthesized with the help of the Center for Nanophase Materials Sciences’ Peter Bonnesen. When this component was mixed with nickel sulfate, the predicted tetrahedral cage formed with sulfate bound in the center. Radu was unambiguously able to confirm the identity of the structure by single-crystal X-ray diffraction measurements performed in Building 4500-South.
“This represents the first time that guest binding within the cavity of a self-assembled cage was predetermined,” Radu says. “The precise placement of the urea groups is responsible for the remarkably strong sulfate binding, which is on a par with that observed in sulfate-binding protein.”
|
Ben Hay (left) and Radu Custelcean with the X-ray diffractometer they used to confirm their self-assembled sulfate cage experiment. |
Computational tools such as HostDesigner are enabling researchers to test solutions to tricky problems at a faster rate. Without the software, the project would have probably taken years of tedious trial-and-error experimentation. “HostDesigner allowed us to get the cage on the first attempt,” Ben says.
Ben is now working on an LDRD project to expand the fragment library used by HostDesigner. “We used only 8,000 fragments to design the sulfate cage. The expanded library contains over 900,000 fragments,” he says.
With this increased level of complexity, he is now investigating adapting HostDesigner to run in parallel on the Jaguar supercomputer.
In principle, HostDesigner can be applied in any instance where one wants to precisely position molecular components in space. Along this line the team is now applying the code to the design of three-dimensional crystalline materials.
The sulfate cage research was supported by the Office of Basic Energy Sciences and the Center for Nanophase Materials Sciences.—B.C. ![]()
 Summer forecast: Lots of building up, tearing down
Summer forecast: Lots of building up, tearing down
|
Cleanup and demolition of Building 3026, a former radioisotope processing facility, will block Central Avenue for much of the rest of the year. |
Lab staff members this summer will find themselves coexisting with a flurry of construction and demolition work on a scale not envisioned just a few months ago.
The work, fueled by funding from the American Recovery and Reinvestment Act, will result in an accelerated schedule for the new Chemical & Materials Sciences Building and the long-anticipated cleanup of disused facilities in the central campus, which will clear the way for reuse of some ideally located brownfields.
Facilities & Operations Directorate staff members have been meeting to carefully coordinate the different projects as they keep an eye on a potential influx of R&D-related work, also catalyzed with stimulus funding. Last month, for example, the Lab won two Energy Frontier Research Center proposals and is partnering on 11 others.
“Our F&O coordination team is focused on understanding the impacts of project requirements on operations, with particular focus on the central campus, which is being managed by multiple prime contractors,” says F&O’s Jim Serafin, who has been tasked with coordinating the many critical interfaces such as road closures, pedestrian re-routes and construction truck traffic .
“One of our biggest challenges is ensuring that as projects evolve and changes are made to scope and schedules, the impacts of these changes on day-to-day Lab operations are understood and well-communicated. Our team meets monthly to review changes to project plans, construction schedules and critical interfaces between projects and operations,” Jim says.
The Chemical & Materials Sciences Building will provide three stories and 160,000 square feet of new laboratory space for researchers currently housed in the 4500 complex. Construction kicks off this month, and ARRA funding has moved the completion up to the spring of 2011.
The new, “shovel-ready” lab building isn’t the only new construction. Work has begun at the west campus, behind Building 1503, on three new greenhouses that will serve the BioEnergy Sciences Center and multiple agencies’ growing bioenergy R&D portfolios. The Advanced Microscopy Laboratory, near the High Temperature Materials Laboratory, is about to double in size with a mirror-image annex to make way for new, state of the art microscopes that will require the facility’s’ specialized interference-proof construction.
Awaiting approval is a proposed project to join the Computational Sciences Building and Engineering Technology Facility with an office annex to accommodate new staff expected to arrive with the Lab’s growing research programs.
Farther west, in the central campus, the order of the day will be what comes down. On Central Avenue between Third and Fifth streets, an old isotope processing facility, Building 3026, will be cleaned up and eventually demolished, the first and one of the tougher jobs in an eventual clearing of problematic old buildings that constituted the original ORNL.
The central campus cleanup is a monumentally complex project that will go far beyond the end of the year. |
“We have a unique opportunity to jumpstart the Integrated Facilities Disposition Project that will ultimately clean up the central campus,” says the Environmental Management Program Office’s Dirk Van Hoesen, who has been planning the central campus cleanup for several years.
In a “Safety Snapshot” video, Dirk notes that with a presence of legacy contamination and other hazards such as asbestos and PCBs in the old facilities and the proximity of staff around the centrally located sites, “it is imperative that this work be done safely.” All work, Dirk says, will be performed under Integrated Safety Management guidelines and procedures.
The Building 3026 project’s largest impact on Lab staff members could be the closing of Central Avenue this summer, possibly through most of the rest of 2009. Sporadic Central Avenue closures are also planned for the Chemical & Materials Sciences Building project and an underground line excavation on the same block.
Those closures and the closing of White Oak Avenue behind the Computational Sciences Building and Engineering Technology Facility for construction on those privately funded buildings will limit alternatives for navigating from one end of the Laboratory to the other for motorists and pedestrians alike, pretty much for the rest of the year.
Another major summer demolition project, although more off the beaten path, is the teardown of the eastern half of the notorious 2000 complex, located at the top of Third Street. Generally referred to as the Quonset huts and dubbed the “Winter Palace” in 2000 by the UT-Battelle transition team, any notion of South Pacific charm has long since been replaced by peeling paint, deteriorated infrastructure and leaky roofs. The eventual clearing of that site will make way for the Oak Ridge Science & Technology Park now springing up just to the northwest.
The central campus cleanup, or IFDP, is a monumentally complex project that will extend far beyond the end of the year. Although stimulus funding has provided a welcome jumpstart, the work could last for decades. Some deteriorating landmarks will go out in the near term, however.
One of them is Building 3550, the paint-flaked World War II-era green wooden structure on Central Avenue that is one of the last examples of an original Manhattan Project structure. It is slated for demolition in FY 2010, as is the 1943-vintage Building 2517, also known as “the little red schoolhouse” for its use as a training facility.
Also slated for demolition work in FY2010 are Building 2026, the decommissioned hot cell facility; Building 2018, currently F&O’s electric & A/C service center; and Building 2011, the original ORNL steam plant including its smokestack. Lance Mezga, who is coordinating D&D projects in that area, says different methods to bring the stack down are being considered. Depending on what’s decided, that could be worth watching.
The old ORNL Cafeteria was torn down last year; only the slab remains. Its removal is also in the IDFP plan. Other structures that have already left the scene in that area include buildings 2506 and 2013, both of which have spontaneously evolved into parking lots.
|
The Flagpole parking lot, save for a sole van, sits emptied for the construction start on the Chemical & Materials Sciences Building. |
Speaking of parking, the Chemical & Materials Sciences Building replaces the Flagpole parking lot, which provided some of the only parking near the 4500 complex until it closed on June 1. There is space in perimeter lots to pick up for the nearly 200 lost general parking slots from Flagpole. Parking coordinator George Baber has addressed the problem of replacing “specialty” parking spaces, such as disability, carpool and government vehicle slots, by redesignating spaces in the area to accommodate those needs. They are outlined in a new Parking Website, portal.ornl.gov/sites/fo/fd/pjm/p/.
Parking lot expansions are planned for the Conference Center lot (136 spaces) and for the North parking lot along Bethel Valley Road (35 spaces). Also in the planning stage is a parking garage, on a site yet to be determined.
In the meantime, for ORNL staff members, ORNL Director Thom Mason’s advice in a recent message to staff is worth repeating: “Please be mindful of advisories and heed the signage around the work areas.”—B.C. ![]()
 ASTM International nuclear committees convene at ORNL
ASTM International nuclear committees convene at ORNL
Two committees of ASTM International, which develops standards for a range of global industries, are meeting this month at ORNL, June 8-11. Committees C26 on the Nuclear Fuel Cycle and E10 on Nuclear Technology and Applications are responsible for a range of standards related to the nuclear industry.
The meeting’s approximately 70 attendees are hosted by the Materials S&T Division. MSTD’s Roger Stoller, currently vice-chairman of the ASTM International board of directors, will serve as board chairman in 2010.
ORNL’s prominence in ASTM International reflects the Lab’s historic and continuing leading role in nuclear materials research and nuclear engineering. Currently more than 20 ORNL researchers participate in ASTM committees, with more participating in ASTM activities without being formal committee members.
The two committees meeting at ORNL provide substantial support to the safe operation of civilian nuclear technology and the radiation processing industry. ASTM standards from a board comprising the industrial, academic and scientific research communities are referenced in regulatory documents throughout the nuclear industry. The committees’ emphasis on technical consensus toward the development of standards that promote best practices throughout the nuclear industry.
The Nuclear Fuel Cycle Committee, with a current membership of approximately 200, has jurisdiction over more than 160 standards playing a preeminent role in all aspects important to the nuclear fuel cycle, including nuclear fuel specifications, spent nuclear fuel, waste materials and repository waste packaging and storage.
The Nuclear Technology and Applications Committee, with a current membership of 210, is responsible for more than 100 standards dealing with all aspects of the nuclear industry except the fuel cycle including irradiation processing, radiation dosimetry for commercial and research purposes, nuclear structural materials, and decontamination and decommissioning.
ORNL staff who are active members of the Nuclear Fuel Cycle or Nuclear Technology and Applications committees include
Roger Stoller, Randy Nanstad, Mikhail Sokolov, Thak Sang Byun, Jy-An Wang, Igor Remec and Doug Selby.
Both committees, which have significant international representation, actively work to maintain a close relationship with DOE and the Nuclear Regulatory Commission.—B.C. ![]()
|
Hummingbirds have returned to the Research Office Building’s courtyard for the summer. The feeders are maintained by F&O’s Pam Golden. |
 Solar Initiative would tap Lab’s R&D resources
Solar Initiative would tap Lab’s R&D resources
Governor Phil Bredesen on May 13 proposed the Volunteer State Solar Initiative, a comprehensive solar-energy and economic-development program that will tap materials science and supercomputing resources at ORNL.
In announcing the new initiative, Gov. Bredesen was joined by legislative leaders and key partners including ORNL, the Tennessee Valley Authority and the University of Tennessee. The solar energy and economic development program will use up to $62.5 million in federal American Recovery and Reinvestment Act funds. The plan is subject to approval by DOE and the state legislature.
The solar institute would be located at the state-funded Joint Institute for Advanced Materials, which will be located on the University of Tennessee campus. Gov. Bredesen also cited the availability of ORNL’s “existing world-class DOE research assets including the Spallation Neutron Source and the world’s most powerful supercomputers.”
“Tennessee is taking advantage of a unique opportunity to become a national leader in the solar industry. By leveraging all of the state’s assets on the single goal of making solar energy more affordable, there is a good chance that the Solar Institute will help bring even more jobs to Tennessee,” says Lab Director Thom Mason.
The initiative also includes a solar farm that will be constructed in Haywood County in west Tennessee. The five-megawatt facility, a partnership with TVA, will be located in an industrial center near Interstate 40.
The solar initiative is the most recent in a series of energy-related investments the state of Tennessee has made over the past two years, including a significant bioenergy initiative. At ORNL, the state has funded three joint institutes, two of which, for scientific computing and biological sciences, have been completed. Construction of the Joint Institute for Neutron Sciences has begun adjacent to the Spallation Neutron Source on Chestnut Ridge.
“Short-term, these new projects will go hand-in-hand with creating or supporting jobs in construction, manufacturing and installation, and scientific efforts to improve the affordability and efficiency of solar energy. Long-term, they will strengthen Tennessee’s reputation as a national energy research hub and emerging force in the U.S. solar industry,” Gov. Bredesen says.![]()
 |
 Shovel-ready’ C&MS Building begins
Shovel-ready’ C&MS Building begins
It might have been a good omen that, as rain fell all around the region, the clouds parted May 27 for the dedication of a major new building project on the ORNL campus
.
Rep. Lincoln Davis and ORO Manager Gerald Boyd joined Lab Director Thom Mason in breaking ground for the new Chemical & Materials Sciences Building. The facility might be the nation’s first “shovel-ready” project to commence, supported by American Recovery and Reinvestment Act funding.
“We’ve had plans to replace this building going back many years now. Because of hard work that’s been done in laying out those plans, we were very well positioned to take advantage of the opportunity to accelerate this project,” Thom told the crowd that gathered under the oaks on the east end of the site.
Thom noted the problems with lab facilities in Buildings 4500-North and South, which after a half-century of service are expensive to maintain and no longer suited to 21st Century science.
The new building’s 56 labs will be served by a central service corridor, a design efficiency that is virtually impossible with the 4500-complex’s multiple L-shaped design.
The exterior will contain the office corridor, which will benefit from natural daylight and what architect Rich Bacino describes as a “nice office environment.”
“We think it’s going to be a perfect place for our science and energy research to be conducted,” says Associate Lab Director Michelle Buchanan, whose Physical Sciences Directorate staff will make up most of the up to 300 residents.
 SNS: Reliability trumps milestones
SNS: Reliability trumps milestones
|
| The neutron scattering school students and hosts: Keeping the SNS running for them. |
All bets are off: The crew at the Spallation Neutron Source has stopped speculating on when its target will fail. The unique unit—the first mercury target—has outlasted even the most optimistic failure predictions. It has been plugging along since late April 2006, when the SNS saw its first neutrons.
The thinking was that sometime last summer or fall the constant bombardment of protons—at a force similar to being spattered with 50-caliber machine-gun rounds 60 times a second—would result in a breach of the cooling water jacket around the circulating mercury.
That hasn’t happened, possibly because the SNS has been ramping up more slowly than originally anticipated. SNS Operations Director Frank Kornegay says now the plan is to change the target out during the scheduled outage next month.
“By then the outer shroud, which surrounds the cooling water for the target, will reach the limits we established,” Frank says. “It exceeded our expectations, ran longer than anybody thought it would.”
The SNS power has reached as high as 850 kilowatts, over halfway to its peak 1.4 megawatts. The megawatt mark seemed reachable in late May, but the failure of a couple of high-voltage components—which is not unusual—combined with the arrival of students with the National School of Neutron and X-ray Scattering made reliability the preferred option.
“We wanted it to be running for them,” Frank says.
 I&C history debuts
I&C history debuts
In 1993, when ORNL turned 50, the Lab marked the occasion by publishing a number of division histories. The history of the old Instrumentation & Controls Division took a little more time, but it’s worth the wait.
Principal author Ray Adams has wrapped up what must have been a labor of love with more than 300 pages describing the division’s accomplishments over its half century.
Beyond the Edge of Technology: Fifty Years of Instrumentation and Controls at ORNL describes, project by project, how I&C kept pace in providing the hardware—such as reactor control systems and signal processing technology—as ORNL expanded from its Manhattan Project beginnings into the largest nonweapons national laboratory. Particularly detailed is the development of the fountain-pen-sized personal radiation monitor in the late 1950s, which was a groundbreaking step toward miniaturization of sensors and detectors.
I&C’s role in ORNL’s early computing ventures, including ORACLE, is also detailed, including the familiar-sounding fact that the early computer’s installation included 40 tons of air conditioning to cool its vacuum tubes.
Also included are personal anecdotes about some sometimes quirky employees (one fellow reported to work with multiple abrasions after attempting to give his cat a bath by stepping into the shower with it).
Ray and his co-authors Dick Anderson, Don Miller and Les Oaks presented a copy to Lab Director Thom Mason and to Ken Tobin, a former I&C Division member who now directs one of I&C’s successor organizations, the Measurement Science & Systems Engineering Division.
Reported by Bill Cabage |
 New ice cage for gas hydrates
New ice cage for gas hydrates
Neutron analysis could boost understanding of a potential ‘fire and ice’ energy source
|
A better molecular-level understanding of gas hydrate structure could open the way to an important new energy source. |
Some say the world will end in fire/Some say in ice,” wrote American poet Robert Frost in “Fire and Ice,” in which fire and ice are metaphors for love and hate respectively. Many are fascinated by fire and ice, possibly because the two represent potentially deadly temperature extremes like those found in our sun and in Earth’s polar regions. For others fire and ice symbolize climate change.
“Fire in the Ice” is the title of a newsletter supported by DOE to promote the National Methane R&D Program. Chris Tulk, an instrument scientist with ORNL’s Spallation Neutron Source, has found the frozen water compounds known as methane hydrates fascinating partly because, if exposed to a lit match during decomposition, the natural gas trapped in ice cages explosively leaps up as a flame. As a result, the “ice” appears to burn, creating an image of abundant energy for an energy-starved world. But what fascinates Chris even more is the discovery in his research group of a new structure—the fourth low-pressure structure ever observed—for the ice cages in gas hydrates.
Methane hydrates, generally known as gas hydrates, are found on the ocean floor and in the subsurface of the permafrost of both the Arctic region and Antarctica, and particularly in the Northwest Territories of Canada. Some scientists estimate that the hydrocarbon resources trapped in these gas hydrates nearly equal the amount of carbon in Earth’s terrestrial biosphere. Thus, gas hydrates are often viewed as a potential solution to future energy shortages.
Because carbon dioxide is known to form gas hydrates, it may be possible to pump CO2 to the ocean floor to displace methane from its ice cages, freeing them to capture and sequester the CO2. The liberated methane would be captured and brought to the surface for use as fuel. The trick would be to understand enough of the mechanism at the atomic and molecular level to make this process viable and safe on a large scale.
Pressure plays a role in hydrate formation, stabilization and transformation to other high-pressure crystalline polymorphs. Chris, who is the instrument scientist for the Spallation Neutrons and Pressure (SNAP) beam line at the SNS, thinks that such research on this water-rich compound is a natural fit for neutron scattering from samples placed under hydrates’ “natural” pressure and temperature conditions, where these materials often form and transform. He believes that the SNAP beam line positions ORNL researchers and other users at SNS to make a large contribution to the molecular-level understanding of the behavior of hydrates and similar materials.
“Gas hydrates must be under pressure to be stable,” Chris says. “Gas hydrates are found deep in ice cores pulled up by drilling in the permafrost. The hydrate cage structure decomposes when the pressure decreases, and the methane molecules escape, leaving behind only ice or water.”
Chris started his research on gas hydrates as a postdoctoral fellow at the Steacie Institute for Molecular Sciences at Canada’s National Research Council. NRC has a long and storied history of research on clathrate inclusion compounds and water-related substances. Chris continued his research on water after joining SNS, where he is currently interested in studying crystallographic transformations in clathrate hydrates as the pressure on these materials is raised to tens of kilobars.
“We began a study at NRC to investigate the structure of guest clusters in high-pressure polymorphs,” Chris says, explaining that polymorphs are solids that have the same chemical composition but different crystal lattice forms. “We decided to use noble-gas atoms like xenon as guest species, because they are much simpler structurally than chemical compound guests and they have comparatively more electrons, thus increasing the weighting of the guest relative to the host. The weight contrast is important for X-ray diffraction studies because X rays are scattered by electrons.”
The team conducted their first experiments on xenon hydrates, which possess the same crystallographic form as methane hydrates. Following pressure quench recovery, Chris and his collaborators found some unexpected results. The choice of xenon also allowed the researchers to perform 129Xe nuclear magnetic resonance spectroscopy, a technique for which NRC is world-renowned that is a very powerful probe of local clathrate structure.
“We froze water and xenon into the well-known hydrate sI structure using a pressure vessel in a freezer,” Chris says. “Then using liquid nitrogen, we temperature-quenched the material to 77 Kelvin and recovered the sample at one atmosphere. After cold-loading this sample into a large pressure cell fitted to a hydraulic ram, we pressurized the material to between 15 and 20 kilobars at room temperature, producing what we thought was the high-pressure polymorph. Our idea then was to quench recover this sample for additional ex situ studies.
“After collecting X-ray diffraction data from these samples, we quickly realized that we had not, in fact, recovered the expected high-pressure form, but rather something different,” Chris says. “We knew we had not decomposed that sample to ice and xenon. That was clear. But the structure of this hydrate was a complete mystery. The problem sat unsolved for well over a year.”
In early 2008 Chris went to the University of Tennessee library in Knoxville. While browsing among the books, he experienced a moment of serendipity.
“By dumb luck I stumbled on a book that I had studied during my postdoctoral time at NRC,” he said. “I flipped through the pages and found a chapter on gas hydrates that contained a table of real and possible hydrate structures. I saw the high- pressure structure that we were expecting to produce, followed by an old structure that was postulated based on polyhedral stacking and that was previously thought to form from bromine hydrate.
|
Chris Tulk |
“I knew that structure was postulated based on composition and not on a structural study. Furthermore, I quickly realized that the bromine hydrate structure was discounted many years ago because it had never been observed. I began to wonder if we had in fact produced the first samples having that structure.
“I copied the information and gave the problem to Ling Yang, a new postdoctoral researcher at ORNL. Within a day Ling returned the verdict that the largely forgotten structure in the book provided a remarkably good fit to our data. The synthesis of this structure was repeated and further NMR experiments confirmed our structural interpretation.”
According to Chris, at low pressure, the water molecules form cages, and each cage traps one xenon molecule. As the pressure increases, a high-pressure polymorph is formed. At high pressure hydrogen water bonds break and re-form such that each of the ice cages rearranges slightly, with some becoming large enough to contain several guest atoms or molecules.
Release of the pressure then results in significant repulsion between guest atoms having like charges in these clusters. As a result, the large cages are torn apart. Completely new and as yet unobserved smaller cages that each contain only one xenon atom are formed. Simulations performed at NRC validated the new hydrate structure measured by NMR and X-ray and neutron diffraction.
These findings were reported in a paper submitted to the Proceedings of the National Academy of Sciences. In additional studies, the researchers found that, at very low temperatures, the guest molecules no longer sit in the centers of the cages. They move off center. Where a guest molecule will sit from one cage to the next is random. This finding was reported in a paper submitted to Physical Review Letters.
Is there a pressure limit for gas hydrates? “Our evidence indicates that exceeding 30 kilobars will cause the hydrate to decompose into xenon and ice,” Chris says. “We learned that water in hydrates makes eight phase transitions before decomposing into ice.”
Chris and his colleagues plan to do experiments with gas hydrates at SNAP. Chris concedes that placing a hydrate sample in an SNS instrument is challenging because the sample either must be kept very cold using liquid nitrogen or kept under pressure for a long time to be stable for neutron scattering studies.
Much has been learned about fire. However, by combining neutron diffraction and computer simulations, perhaps even more may be learned about water and ice.—Carolyn Krause ![]()
 ORNL People
ORNL People
According to an independent analysis of the field of high-temperature superconductors conducted by ScienceWatch.com, the Materials S&T Division’s Amit Goyal ranks number one worldwide in the total number of citations and number four worldwide in the total number of papers published during the last decade (1999-2009). Other ORNL researchers made the lists, including Elbio Dagotto, Dave Christen, M. Paranthaman, Dominic Lee, Ron Feenstra, Eliot Specht, Claudia Cantoni, Don Kroeger, Fred List and the late Patrick Martin.
The first publication presenting data from the Bio-SANS instrument at the High Flux Isotope Reactor has been published, in the April 1, 2009, issue of Chemical Engineering Journal. Gary Baker of the Chemical Sciences Division and William Heller of the Center for Structural Molecular Biology used small-angle neutron scattering to investigate the effect of solutions containing ionic liquids on protein structure and oligomerization state.
A Physical Review Letters article co-authored by the Neutron Scattering Science Division’s’s Gang Cheng and Yuri Melnichenko was identified as an “editor’s suggestion,” indicating high importance. Working with Princeton’s William Graessley, Gang and Yuri collected data using the General Purpose SANS at HFIR and a SANS instrument at NIST. Their findings revealed a distinct crossover in polymer structure from semidilute to concentrated solutions.
Karsten Steinhaeuser, a post-master’s degree associate at the Lab and concurrently completing his doctorate at the University of Notre Dame, won the best poster award for his doctoral forum presentation at the SIAM International Conference on Data Mining. Karsten’s work was based on an ongoing Laboratory Directed R&D project in the area of climate extremes and uncertainty.
UT-Battelle received the Innovator Award, given to a business that has developed a new technology, innovative product or service or applied a business system or service in an innovative way, at the Knoxville Chamber’s annual Pinnacle Awards. The chamber cited the construction of the Spallation Neutron Source and the growth of supercomputing at ORNL. ![]()
 Kiffin to kick off United Way drive
Kiffin to kick off United Way drive
|
Kiffin |
The 2009 United Way Campaign kicks off in June with University of Tennessee head coach Lane Kiffin and other Vol football coaches in a personal appearance on June 23.
A competition dance team from the Oak Ridge Academy of Dance and several members of the UT-Dance Team will perform before coach Kiffin and his coaching staff officiate at the finals of a football throwing competition.
Campaign chair Edgar Lara-Curzio, co-chair Kathy Carney and the ORNL United Way Cabinet volunteers are working hard to make 2009 one of the top United Way campaigns.
This year ORNL’s more than 4,000 retirees will be able to contribute to the campaign. Based on the feedback from retirees on United Way-supported programs such as Lifesaver provides bracelet transmitters to people who suffer Alzheimer’s disease and other disorders, Edgar and Kathy believe the campaign response from them will be very positive.
This year’s agency tours for United Way reps include the Keystone Adult Day Care Center, the Emory Valley Center and Group Home and the Boys and Girls Club of Oak Ridge.
“Sometimes we operate inside a cocoon at ORNL, unaware of the struggles of people in the community,” says Edgar. “These visits are an opportunity to learn how contributions to United Way are being utilized to make a difference in the community.”
The International Festival, one of the Lab’s most popular events for the United Way, is scheduled for August 6. ![]()
 New Staff Members
New Staff Members
May 2009
Nasr Eldine Mohammed Alkadi, Joshua Ryan New and Steven Lawrence Campbell, Energy & Transportation Science
George Patrick Andrews, Energy & Engineering Sciences Dir.
Bruce Edward Bates (re-instatement), Robert Alexander Lefebvre and Robert Timothy Collins, Nuclear Science & Technology
Brian Keith Goins (re-hire), Facilities Management
David Rutledge Grant and Darren Eugene Norris, Facilities Development
John Daniel Kennedy Rivard, National Security Dir.
Natalie Reagan White, Channa Katrina Palmer and Joshua Mark Scull, Human Resources Dir.
Felicia Dair Bellamy-Roseberry (re-hire) and Jagjit Nanda, Materials Science and Technology
Holly Geneace Garrett (re-hire), Andrew Edward Lyles (re-hire), Anthony Dewayne Green (re-hire), Brittany Nicole
Kilburn, Derek Steven Townsend, Jason Leroy Futrell (re-hire) and Billy Wayne Wood, Campus Support & Instrumentation
Robert Ray Grayson, Jr., Debra Lynn Alexander, William Scott Alred, Dianne Margaret Bannen and Michael Wilder Hittman, Fabrication, Hoisting & Rigging
Danny Roger Honeycutt (re-hire), Office of Chief Information Officer
Michael Alan Matheson, Center for Computational Sciences
Gina Ann O’Brien, Environmental Sciences
Mark Anthony Sanschargrin, Darrel Allen Lively and Jeffrey Claude Patterson (re-instatement), NScD Research Accelerator
Mike A. Harper, Environmental Management Program Office
Crystal Rae Hoffmann, Safety Services
Bo Liu, Computer Science and Mathematics
Linda Joyce Paschal, Global Nuclear Security Technology
Jacob Hunter Phillips (re-hire), Amanda Renee Presley and Jonathan Paul Thompson, Contracts
Andrew Wayne Reeves and Latecea N. Brock, Business & Information Services Dir.
Craig Aaron Shue, Computational Sciences & Engineering
Xiao-Guang Sun, Chemical Sciences
Chris Bensing Boehnen, Measurement Science & Systems Engr
Matthew Christopher White, Audit and Oversight Dir.
 Wellness
= fun
Wellness
= fun
|
 |
|
Participation in this year’s HealthFest increased by more than half, says wellness coordinator Joan Lawson. That might have been because of all the fun events, including a water balloon toss (above), a sponge race (far right, with Wes Goddard) or “pin the note on Thom” (right, with Jennifer Haun). Or maybe it was the “reward points” for participating, which for salaried staff can add up to a medical premium discount.
ORNL’s wellness activities continue throughout the year.
“Physical activity is the best medicine you can take for your health,” says Joan. ![]()