- Number 382 |
- February 18, 2013
The motor that drives Archaea
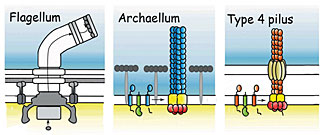
The motile structures in Bacteria and Archaea. The
archaellum (center) functions like a bacterial flagellum
but its structure resembles a bacterial Type 4 pilus.
The protein structure of the motor that propels Archaea – the third domain of life, along with Bacteria and Eukarya – has been characterized for the first time by a team from DOE’s Lawrence Berkeley National Laboratory and Germany’s Max Planck Institute for Terrestrial Microbiology.
The researchers, led by Sonja-Verena Albers of the Max Planck Institute and John Tainer of Berkeley Lab’s Life Sciences Division and the Scripps Research Institute, call this unique motor an archaellum. It functions like a bacterial flagellum, a whip-like rotating propeller, but structurally it more closely resembles the Type 4 pilus, the filamentary “grappling hook” by which bacteria attach to surfaces and pull themselves along.
Genetic modifications of the extremophile S. acidocaldarius in the Albers lab pinpointed the FlaI (“flah-eye”) protein as responsible for assembling the archaellum and making it rotate. Sophia Reindl of Tainer’s lab crystallized the protein and used beamline 8.3.1 at Berkeley Lab’s Advanced Light Source (ALS) to do x-ray crystallography, which revealed that FlaI consists of two parts, a globular base connected to a moveable tip by a flexible linker.
FlaI is an ATPase, an enzyme that releases energy by binding to adenosine triphosphate, ATP, and hydrolyzing it to adenosine diphosphate, ADP. “If you can find different orientations between the bound states of ATP and ADP, you can assume the protein is performing a certain movement,” Reindl says.
But while she could crystallize FlaI bound to ADP, FlaI bound to ATP was impossible to crystallize. She turned to small-angle x-ray scattering, SAXS, at the SIBYLS beamline at the ALS. “Without a crystal we couldn’t get atomic resolution,” Reindl says, “but in some ways the advantages were greater, because we could see the overall conformation of the protein in a more normal solution state.”
The different conformations revealed by combining the crystallographic and SAXS data showed that when FlaI proteins are bound to ATP they arrange themselves into six-unit rings resembling a crown, forming a circlet with flexible points. The crown both assembles the archaellum and causes it to rotate.
As an individual FlaI unit in the ring processes ATP and reduces it to ADP, the whole unit moves upward, pushing up the growing filament and opening a gap through which additional subunits are added to its base – much like the mechanism in a bacterial Type 4 pilus, although the resulting structures operate very differently.
Archaea may be important players in the microbiota of the human gut, and since Type 4 pili are responsible for pathogenicity in many bacteria, including deadly strains of E. coli, analyzing the similar structure of the archaellum will help scientists understand how archaea interact with human cells.
The research team’s biggest remaining challenge is to learn how the movement of individual FlaI units is transferred to the rotation of the archaellum filament. Does the whole FlaI crown rotate, or only the archaellum? If so, how? While many questions remain, the MPI-Berkeley Lab collaboration has already produced the first and only in-depth structural study of an archaellum protein, another step toward solving the mysteries of Archaea.[Paul Preuss, 510.486.6249,
paul_preuss@lbl.gov]
