- Number 406 |
- February 3, 2014
Disordered materials hold promise for better batteries
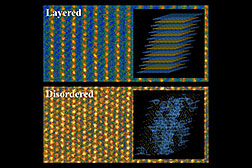
Conventional layered lithium and transition
metal cathode material (top) and the new
disordered material studied by researchers
at MIT and Brookhaven (bottom) as seen
through a scanning tunneling electron
microscope. Inset images show diagrams
of the different structures in these materials.
(In the disordered material, the blue lines
show the pathways that allow lithium ions
to traverse the material.)
Lithium batteries, with their exceptional ability to store power per a given weight, have been a major focus of research to enable use in everything from portable electronics to electric cars. Now researchers at Massachusetts Institute of Technology (MIT) and DOE's Brookhaven Lab have found a whole new avenue for such research: the use of disordered materials, which had generally been considered unsuitable for batteries.
In a rechargeable lithium-based battery, lithium ions — atoms that have given up an electron, and thus carry a net charge — are pulled out of the battery's cathode during the charging process, and returned to the cathode as power is drained. But these repeated round-trips can cause the electrode material to shrink and expand, leading to cracks and degrading performance over time.
In today's lithium batteries, those cathodes are usually made of an orderly crystalline material, sometimes in a layered structure. When slight deviations from that perfect order are introduced, the battery's efficiency generally goes down — so disordered materials have mostly been ignored in the search for improved battery materials.
But it turns out this correlation is far from universal: Certain kinds of disorder can provide a significant boost in cathode performance, the researchers have found through a combination of computer modeling and laboratory experiments. These surprising findings were reported in the journal Science.
Scientists had thought layering was necessary to provide a pathway—a channel with nothing in the way— for lithium to pass in and out of the cathodes, and that disorder significantly reduced lithium ion mobility. But it turns out that a significant excess of lithium in the material the scientists studied changes things dramatically. Though excess lithium produces irregular pathways, it turns out that these nevertheless can still act as efficient channels for the lithium ions. And the material offers an extra bonus: While the irregular channels let lithium pass just as easily as it does in a layered material, in the disordered material the lithium ions don't push the layers out of shape.
This research shows a new direction the researchers can take in searching for even better materials, opening a whole new category of possibilities that had previously been ignored in battery design.
Based on a story by David L. Chandler, MIT News Office[Karen McNulty Walsh, 631.344.8350,
kmcnulty@bnl.gov]
