- Number 339 |
- June 13, 2011
PNNL applies pressure to solving global climate change issues
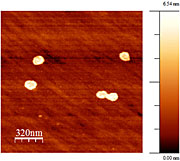
The new high-pressure atomic
force microscope reveals tiny
details of in situ reactions, such
as the decompositions shown
here on the mineral surface.
Scientists have been under pressure to help solve global climate change by reducing greenhouse gas emissions through methods such as carbon sequestration, the capture and long-term storage of carbon dioxide in deep geological formations. Now scientists at DOE’s Pacific Northwest National Laboratory, with colleagues from Wright State University and Lawrence Berkeley National Laboratory, have taken a step closer with the development of a high-pressure atomic force microscope.
Located at DOE’s Environmental Molecular Sciences Laboratory, this new AFM provides researchers the means to observe in real-time mineral transformation processes arising from reaction with supercritical carbon dioxide at the atomic scale. These transformations occur under pressures and temperatures similar to those needed for carbon sequestration in geological formations.
Capturing carbon: Injecting captured CO2 underground in porous host rock to depths of greater than half a mile subjects it to temperatures and pressures that allow it to be maintained in a supercritical fluid state. A supercritical fluid has a mixture of the properties of a liquid and a gas. Researchers are seeking to better understand the chemical interactions of the supercritical CO2 with various minerals found in potential host rocks for geologic sequestration.
One tough microscope: Previous instrumentation required researchers to depressurize reaction cells and capture only snap shots in time before and after, but not during the reaction, altering experimental conditions between images. With the ability of the new AFM to achieve pressures of 100 atm, or atmospheres, and temperatures up to ~350K, they now can capture an entire movie in real-time of mineral surfaces as they change in supercritical CO2 at the level of single atomic steps. Previous systems only handled pressures reaching 50 atm, not great enough to attain the minimum pressures needed to achieve the supercritical state of CO2.
“This new AFM provides unprecedented in situ access to interfacial phenomena at solid-fluid interfaces under pressure,” says Dr. Kevin Rosso, one of the project researchers at PNNL. “This information is key to more fully understanding geochemical processes under a much wider range of geologically relevant conditions—especially those relevant to carbon sequestration.”
Understanding what reactions are taking place, how fast they happen and what the products of the reactions are will aid in the selection of sites for future carbon sequestration operations to help slow the increase of CO2 in the earth’s atmosphere while continuing to allow the use of fossil-based fuels for energy.
Making it happen: The research is being conducted under the PNNL Carbon Sequestration Initiative, a five-year, multi-million dollar investment to advance capabilities in geologic carbon sequestration. The work was performed using EMSL, a national scientific user facility.
The research team was Kevin Rosso, PNNL; Scott Lea, EMSL, PNNL; and co-investigators Steven Higgins, Department of Chemistry, Wright State University, Ohio; and Kevin Knauss, Lawrence Berkeley National Laboratory.
Their research is highlighted in a paper, “A high-pressure atomic force microscope for imaging in supercritical carbon dioxide,” published online April 2011in the AIP Review of Scientific Instruments.Submitted by DOE's Pacific Northwest National Laboratory
