- Number 380 |
- January 21, 2013
An unexpected pairing of frustrated molecules
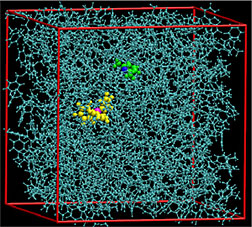
PNNL researchers built simulations showing
how two molecules combine to activate
hydrogen, shedding new light on a reaction
that could, one day, support hydrogenation
for biofuels.
While their shapes frustrate traditional bonding, two unreactive molecules come together and surround themselves within a solvent cage to create a reactive environment and split hydrogen. Researchers at DOE's Pacific Northwest National Laboratory are revealing the role of the solvent in this process. Splitting a hydrogen molecule into a proton and a hydride ion (H-), known as activating the hydrogen, is vital for sustainable energy production and storage. The pair of molecules is called a frustrated Lewis pair.
"Conventional wisdom says that frustrated Lewis pairs should not be able to activate hydrogen—but they do. We wanted to know why," said Dr. Greg Schenter, a theoretical chemist on this project.
Turning plant material or other renewable resources into fuels requires adding hydrogen without taking up excessive energy. This requirement demands an effective catalyst. These studies provide fundamental insights into the processes that could one day be used to create that catalyst.
This research was funded by DOE's Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division.
[Kristin Manke, 509.372.6011,
kristin.manke@pnnl.gov]
