- Number 447 |
- September 7, 2015
Scientists discover precise location of active sites on popular catalyst
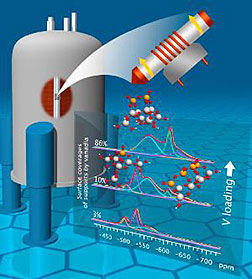
Experimental work with vanadium oxide
catalysts relied on sophisticated
spectroscopy and chemical structure
calculations to establish a relationship
between specific sites on the catalysts
and the degree of activity they bring out
in a reaction.
If you want to change a situation, it's often best to get to the heart of the matter. For chemists working to improve commercially important reactions, this means delving into the active sites on catalysts that speed the reactions behind billions of dollars’ worth of chemicals and other products. Active sites are where the reaction actually happens.
If active sites work slowly or fail quickly, the result is higher costs and lower production rates. To make better active sites, scientists need to see them. For the first time, a team led by researchers at DOE’s Pacific Northwest National Laboratory saw the active sites on well-known vanadium-based catalysts that are important in commercial processes for manufacturing such products as polyesters, sulfuric acid, synthetic resins, epoxy coatings and others.
The team included experts from Washington State University, the University of Alabama, and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Using magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy, they were able to obtain detailed information about the structures of active species of oxides of vanadium as they exist when supported on the surfaces of titanium dioxide.
The scientists correlated the various peaks observed in the NMR spectra with the catalysts' reactivity during an oxidation reaction that removes a hydrogen atom from methanol. Determining which structures do the best job of catalyzing reactions, could help researchers improve control of chemical processes by finding ways to create more of them or protect existing sites from degrading during reactions—or both.[Ken Hodge, 509.372.6121,
Kenneth.Hodge@pnnl.gov]
